ONEXTON Drug Patent Profile
✉ Email this page to a colleague
When do Onexton patents expire, and when can generic versions of Onexton launch?
Onexton is a drug marketed by Bausch and is included in one NDA. There are five patents protecting this drug and two Paragraph IV challenges.
This drug has twenty patent family members in fourteen countries.
The generic ingredient in ONEXTON is benzoyl peroxide; clindamycin phosphate. There are seventeen drug master file entries for this compound. Sixteen suppliers are listed for this compound. Additional details are available on the benzoyl peroxide; clindamycin phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Onexton
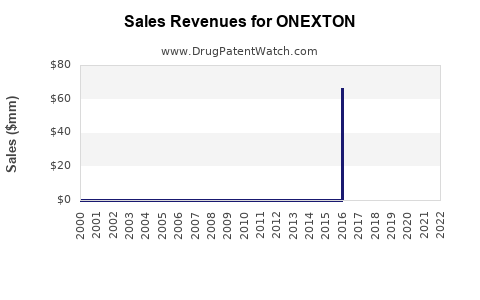
Annual sales in 2022 were $3mm indicating the motivation for generic entry (peak sales were $67mm in 2016).
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ONEXTON?
- What are the global sales for ONEXTON?
- What is Average Wholesale Price for ONEXTON?
Summary for ONEXTON
| International Patents: | 20 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 3 |
| Patent Applications: | 8 |
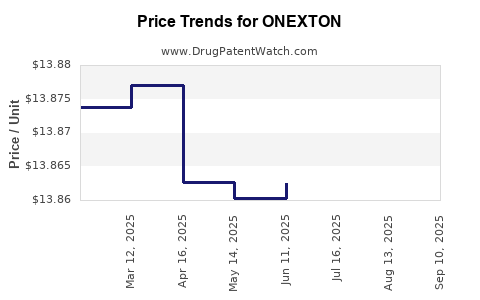
| Drug Prices: | Drug price information for ONEXTON |
| Drug Sales Revenues: | Drug sales revenues for ONEXTON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ONEXTON |
| What excipients (inactive ingredients) are in ONEXTON? | ONEXTON excipients list |
| DailyMed Link: | ONEXTON at DailyMed |
Recent Clinical Trials for ONEXTON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Derm Research, PLLC | Phase 4 |
| Actavis Inc. | Phase 3 |
| Taro Pharmaceuticals USA | Phase 1 |
Pharmacology for ONEXTON
| Drug Class | Lincosamide Antibacterial |
| Physiological Effect | Decreased Sebaceous Gland Activity Neuromuscular Blockade |
Paragraph IV (Patent) Challenges for ONEXTON
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ONEXTON | Gel | benzoyl peroxide; clindamycin phosphate | 1.2%/3.75% | 050819 | 1 | 2015-09-30 |
| ONEXTON | Gel | benzoyl peroxide; clindamycin phosphate | 1.2%/2.5% | 050819 | 1 | 2012-12-20 |
US Patents and Regulatory Information for ONEXTON
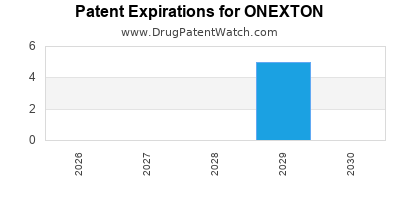
ONEXTON is protected by eleven US patents.
Expired US Patents for ONEXTON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ONEXTON
See the table below for patents covering ONEXTON around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Croatia | P20200450 | ⤷ Get Started Free | |
| Canada | 2723029 | FORMULATIONS PHARMACEUTIQUES TOPIQUES CONTENANT UNE FAIBLE CONCENTRATION DE PEROXYDE DE BENZOYLE EN SUSPENSION DANS DE L'EAU ET UN SOLVANT ORGANIQUE MISCIBLE AVEC L'EAU (TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING A LOW CONCENTRATION OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND A WATER-MISCIBLE ORGANIC SOLVENT) | ⤷ Get Started Free |
| Japan | 6006272 | ⤷ Get Started Free | |
| Mexico | 2010013152 | FORMULACIONES FARMACEUTICAS TOPICAS QUE CONTIENEN UNA BAJA CONCENTRACION DE PEROXIDO DE BENZOILO EN SUSPENSION EN AGUA Y UN SOLVENTE ORGANICO MISCIBE EN AGUA. (TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING A LOW CONCENTRATION OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND A WATER-MISCIBLE ORGANIC SOLVENT.) | ⤷ Get Started Free |
| Spain | 2153377 | ⤷ Get Started Free | |
| South Korea | 20110014651 | TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING A LOW CONCENTRATION OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND A WATER-MISCIBLE ORGANIC SOLVENT | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ONEXTON
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | 122008000041 | Germany | ⤷ Get Started Free | PRODUCT NAME: ADAPALEN IN KOMBINATION MIT BENZOYLPEROXID; NAT. REGISTRATION NO/DATE: 67913.00.00 20080229; FIRST REGISTRATION: DAENEMARK 40440 20071218 |
| 1458369 | C01458369/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: ADAPALENUM + BENZOYLIS PEROXIDUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 58460 19.05.2009 |
| 1586316 | SPC/GB11/054 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: BROMFENAC 2-AMINO-3-(4-BROMOBENZOYL)PHENYLACETIC ACID OR A PHARMACOLOGICALLY ACCEPTABLE SALT THEREOF OR A HYDRATE THEREOF; REGISTERED: UK EU/1/11/692/001 20110523 |
| 1586316 | 122011100019 | Germany | ⤷ Get Started Free | PRODUCT NAME: BROMFENAC (2-AMINO-3-(4-BROMOBENZOYL)PHENYLESSIGSAEURE); REGISTRATION NO/DATE: EU/1/11/692/001 20110518 |
| 0526708 | C300097 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BOSENTAN, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN HYDRAAT OF IN DE VORM VAN EEN ESTER VAN DE HYDROXYLGROEP VAN DE 2-HYDROXYETHOXY REST MET EEN ZUUR MET DE FORMULE R5-OH, WAARIN R5 EEN C1-7-ALKANOYL, BENZOYL, OF HETEROCYCLYCARBONYL VOORSTELT; NATL. REGISTRATION NO/DATE: U/1/02/220/001 - 005 20020515; FIRST REGISTRATION: CH IKS 58841 01 - 02 20020228 |
| 1667986 | 92172 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ONEXTON (Tepotintoin, 0.75%/3%)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
