NUBEQA Drug Patent Profile
✉ Email this page to a colleague
When do Nubeqa patents expire, and when can generic versions of Nubeqa launch?
Nubeqa is a drug marketed by Bayer Healthcare and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and sixty-six patent family members in thirty-nine countries.
The generic ingredient in NUBEQA is darolutamide. One supplier is listed for this compound. Additional details are available on the darolutamide profile page.
DrugPatentWatch® Generic Entry Outlook for Nubeqa
Nubeqa was eligible for patent challenges on July 30, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 27, 2038. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for NUBEQA?
- What are the global sales for NUBEQA?
- What is Average Wholesale Price for NUBEQA?
Summary for NUBEQA
| International Patents: | 166 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 46 |
| Clinical Trials: | 16 |
| Patent Applications: | 1,071 |
| Drug Prices: | Drug price information for NUBEQA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NUBEQA |
| What excipients (inactive ingredients) are in NUBEQA? | NUBEQA excipients list |
| DailyMed Link: | NUBEQA at DailyMed |
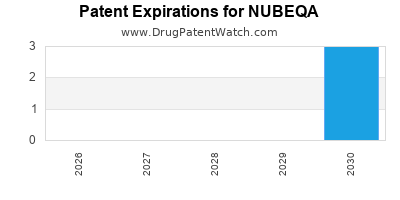

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for NUBEQA
Generic Entry Date for NUBEQA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for NUBEQA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| K36 Therapeutics, Inc. | PHASE1 |
| NRG Oncology | Phase 2 |
| Celcuity Inc | Phase 1/Phase 2 |
Paragraph IV (Patent) Challenges for NUBEQA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NUBEQA | Tablets | darolutamide | 300 mg | 212099 | 1 | 2023-07-31 |
US Patents and Regulatory Information for NUBEQA
NUBEQA is protected by sixteen US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of NUBEQA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
EU/EMA Drug Approvals for NUBEQA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bayer AG | Nubeqa | darolutamide | EMEA/H/C/004790NUBEQA is indicated for the treatment of adult men with- non metastatic castration resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease (see section 5.1).- metastatic hormone sensitive prostate cancer (mHSPC) in combination with docetaxel and androgen deprivation therapy (see section 5.1). | Authorised | no | no | no | 2020-03-27 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for NUBEQA
When does loss-of-exclusivity occur for NUBEQA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18229817
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2019018458
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 55019
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 19002540
Estimated Expiration: ⤷ Get Started Free
Patent: 23002780
Estimated Expiration: ⤷ Get Started Free
China
Patent: 0382467
Estimated Expiration: ⤷ Get Started Free
Patent: 1021396
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0250684
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1992103
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Patent: 59527
Patent: FABRICATION D'UN PRODUIT PHARMACEUTIQUE CRISTALLIN (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 71505
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 57071
Estimated Expiration: ⤷ Get Started Free
Patent: 20510018
Patent: 結晶性医薬品の製造
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 19010452
Patent: FABRICACION DE UN PRODUCTO FARMACEUTICO CRISTALINO. (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7848
Patent: Manufacture of a crystalline pharmaceutical product
Estimated Expiration: ⤷ Get Started Free
Patent: 7194
Patent: Manufacture of a crystalline pharmaceutical product
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 855
Patent: PROIZVODNJA KRISTALNOG FARMACEUTSKOG PROIZVODA (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 92732
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2676383
Estimated Expiration: ⤷ Get Started Free
Patent: 190126111
Patent: 결정성 의약품의 제조
Estimated Expiration: ⤷ Get Started Free
Patent: 240096691
Patent: 결정성 의약품의 제조 (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT)
Estimated Expiration: ⤷ Get Started Free
Patent: 250065940
Patent: 결정성 의약품의 제조 (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 27971
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 6071
Patent: КРИСТАЛІЧНІ ЧАСТИНКИ N-((S)-1-(3-(3-ХЛОР-4-ЦІАНОФЕНІЛ)-1H-ПІРАЗОЛ-1-ІЛ)-ПРОПАН-2-ІЛ)-5-(1-ГІДРОКСІЕТИЛ)-1H-ПІРАЗОЛ-3-КАРБОКСАМІДУ (MANUFACTURE OF A CRYSTALLINE PHARMACEUTICAL PRODUCT)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering NUBEQA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | S2000015 | ⤷ Get Started Free | |
| Spain | 3027971 | ⤷ Get Started Free | |
| Brazil | PI0708036 | método de proteção de material de propagação de planta, planta e/ou órgãos de planta | ⤷ Get Started Free |
| Georgia, Republic of | P20166472 | ANDROGEN RECEPTOR MODULATING COMPOUNDS | ⤷ Get Started Free |
| Denmark | 3592732 | ⤷ Get Started Free | |
| China | 105061313 | ⤷ Get Started Free | |
| Taiwan | 202114658 | Pharmaceutical composition of darolutamide | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NUBEQA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2493858 | 2020018 | Norway | ⤷ Get Started Free | PRODUCT NAME: DAROLUTAMID, EVENTUELT I FORM AV ET FARMASOEYTISK AKSEPTABELT SALT ELLER ESTER DERAV; REG. NO/DATE: EU/1/20/1432/ 20200401 |
| 2493858 | C20200015 00323 | Estonia | ⤷ Get Started Free | PRODUCT NAME: DAROLUTAMIID;REG NO/DATE: EU/1/20/1432 30.03.2020 |
| 2493858 | 20C1028 | France | ⤷ Get Started Free | PRODUCT NAME: DAROLUTAMIDE Y COMPRIS SES SELS PHARMACEUTIQUEMENT ACCEPTABLES ET SES ESTERS COUVERTS PAR LE BREVET DE BASE; REGISTRATION NO/DATE: EU/1/20/1432/001 20200330 |
| 1986495 | 33/2019 | Austria | ⤷ Get Started Free | PRODUCT NAME: SEDAXAN ODER EIN TAUTOMER DAVON, FLUDIOXONIL UND METALAXYL M; NAT. REGISTRATION NO/DATE: 3979-0 20181130; FIRST REGISTRATION: NL 29317 20171229 |
| 1986495 | 19C1035 | France | ⤷ Get Started Free | PRODUCT NAME: COMPOSITION COMPRENANT SEDAXANE OU UN TAUTOMERE DE CE COMPOSE, FLUDIOXONIL ET METALAXYL-M; NAT. REGISTRATION NO/DATE: 2180766 20181218; FIRST REGISTRATION: NL - 15544N 20171229 |
| 2493858 | 2020C/514 | Belgium | ⤷ Get Started Free | PRODUCT NAME: DAROLUTAMIDE OPTIONEEL IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF ESTER DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/20/1432 20200330 |
| 2493858 | PA2020514 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: DAROLUTAMIDAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA ARBA ESTERIS; REGISTRATION NO/DATE: EU/1/20/1432 20200327 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for NUBEQA (Darolutamide): A Comprehensive Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
