NEXTERONE Drug Patent Profile
✉ Email this page to a colleague
When do Nexterone patents expire, and when can generic versions of Nexterone launch?
Nexterone is a drug marketed by Baxter Hlthcare and is included in one NDA. There is one patent protecting this drug.
This drug has twenty-nine patent family members in thirteen countries.
The generic ingredient in NEXTERONE is amiodarone hydrochloride. There are fifteen drug master file entries for this compound. Thirty-one suppliers are listed for this compound. Additional details are available on the amiodarone hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nexterone
A generic version of NEXTERONE was approved as amiodarone hydrochloride by TEVA PHARMS on November 30th, 1998.
Summary for NEXTERONE
| International Patents: | 29 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 111 |
| Clinical Trials: | 3 |
| Patent Applications: | 793 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for NEXTERONE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NEXTERONE |
| What excipients (inactive ingredients) are in NEXTERONE? | NEXTERONE excipients list |
| DailyMed Link: | NEXTERONE at DailyMed |
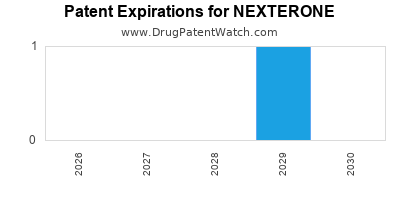

US Patents and Regulatory Information for NEXTERONE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-001 | Dec 24, 2008 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-002 | Nov 16, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-003 | Nov 16, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NEXTERONE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-001 | Dec 24, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-001 | Dec 24, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-002 | Nov 16, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-001 | Dec 24, 2008 | ⤷ Sign Up | ⤷ Sign Up |
| Baxter Hlthcare | NEXTERONE | amiodarone hydrochloride | INJECTABLE;INJECTION | 022325-003 | Nov 16, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for NEXTERONE
See the table below for patents covering NEXTERONE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2771879 | COMPOSITIONS DE CYCLODEXTRINE A GROUPEMENTS ETHER SULFOALKYLIQUE (SULFOALKYL ETHER CYCLODEXTRIN COMPOSITIONS) | ⤷ Sign Up |
| Spain | 2176206 | ⤷ Sign Up | |
| Japan | 2005530744 | ⤷ Sign Up | |
| Japan | 2011116776 | FORMULATION CONTAINING AMIODARONE AND SULFOALKYL ETHER CYCLODEXTRIN | ⤷ Sign Up |
| European Patent Office | 2402008 | Formulations contenant de l'amiodarone et de la cyclodextrine d'éther de sulfoalkyle (Formulations containing amiodarone and sulfoalkyl ether cyclodextrin) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |


