MIRENA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Mirena, and when can generic versions of Mirena launch?
Mirena is a drug marketed by Bayer Hlthcare and is included in one NDA. There are two patents protecting this drug.
This drug has forty-five patent family members in twenty countries.
The generic ingredient in MIRENA is levonorgestrel. There are twenty drug master file entries for this compound. Thirty-four suppliers are listed for this compound. Additional details are available on the levonorgestrel profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Mirena
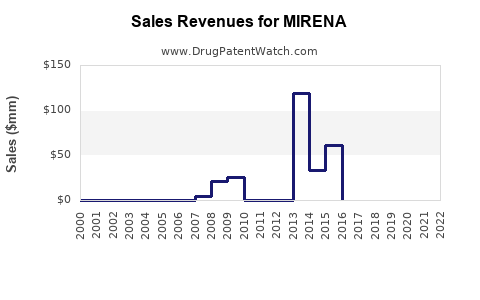
A generic version of MIRENA was approved as levonorgestrel by NOVEL LABS INC on February 22nd, 2013.
Summary for MIRENA
| International Patents: | 45 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 104 |
| Clinical Trials: | 69 |
| Patent Applications: | 4,535 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for MIRENA |
| What excipients (inactive ingredients) are in MIRENA? | MIRENA excipients list |
| DailyMed Link: | MIRENA at DailyMed |

Recent Clinical Trials for MIRENA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Arkansas Children's Hospital Research Institute | Phase 4 |
| Boston Children's Hospital | Phase 2 |
| Kenya Medical Research Institute | Phase 4 |
Pharmacology for MIRENA
| Drug Class | Progestin Progestin-containing Intrauterine System |
| Physiological Effect | Inhibit Ovum Fertilization |
Anatomical Therapeutic Chemical (ATC) Classes for MIRENA
US Patents and Regulatory Information for MIRENA
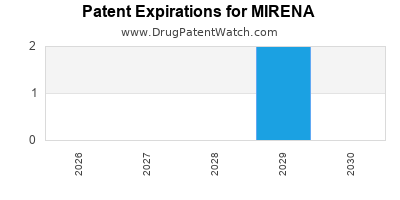
MIRENA is protected by two US patents.
Patents protecting MIRENA
Inserter
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF POSITIONING AN INTRAUTERINE SYSTEM (IUS) BY DETERMINING A DEPTH OF THE UTERUS, HOLDING AN INSERTER HANDLE WITH ONE HAND, INSERTING THE IUS INTO THE UTERUS, AND RETRACTING A SLIDER ON THE HANDLE TO RELEASE THE IUS INTO THE UTERUS
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF PREVENTING PREGNANCY BY PROVIDING AN INTRAUTERINE SYSTEM (IUS), HOLDING AN INSERTER HANDLE WITH ONE HAND, INSERTING THE IUS INTO THE UTERUS, AND MOVING A SLIDER IN THE HANDLE TO RELEASE THE IUS WITHIN THE UTERUS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | MIRENA | levonorgestrel | INTRAUTERINE DEVICE;INTRAUTERINE | 021225-001 | Dec 6, 2000 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Bayer Hlthcare | MIRENA | levonorgestrel | INTRAUTERINE DEVICE;INTRAUTERINE | 021225-001 | Dec 6, 2000 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MIRENA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | MIRENA | levonorgestrel | INTRAUTERINE DEVICE;INTRAUTERINE | 021225-001 | Dec 6, 2000 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for MIRENA
See the table below for patents covering MIRENA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovakia | 282290 | ⤷ Sign Up | |
| Israel | 211058 | מחדיר (Inserter for ius) | ⤷ Sign Up |
| Finland | 945895 | ⤷ Sign Up | |
| Hong Kong | 1005222 | ⤷ Sign Up | |
| Portugal | 2352470 | ⤷ Sign Up | |
| Bulgaria | 101744 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MIRENA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 132016000025143 | Italy | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL ED ETINILESTRADIOLO(SEASONIQUE); AUTHORISATION NUMBER(S) AND DATE(S): 17/0017/15-S, 20150211;042139016, 20150414 |
| 1453521 | 300814 | Netherlands | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1453521 | C201630040 | Spain | ⤷ Sign Up | PRODUCT NAME: ETINILESTRADIOL Y MEZCLA DE LEVONORGESTREL Y ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: 80340; DATE OF AUTHORISATION: 20160122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150211 |
| 1453521 | 122015000093 | Germany | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| 1453521 | 39/2015 | Austria | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1453521 | 15C0050 | France | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


