MAYZENT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Mayzent, and what generic alternatives are available?
Mayzent is a drug marketed by Novartis and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and forty-two patent family members in forty countries.
The generic ingredient in MAYZENT is siponimod. One supplier is listed for this compound. Additional details are available on the siponimod profile page.
DrugPatentWatch® Generic Entry Outlook for Mayzent
Mayzent was eligible for patent challenges on March 26, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 27, 2028. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for MAYZENT
| International Patents: | 142 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 63 |
| Clinical Trials: | 3 |
| Patent Applications: | 309 |
| Drug Prices: | Drug price information for MAYZENT |
| What excipients (inactive ingredients) are in MAYZENT? | MAYZENT excipients list |
| DailyMed Link: | MAYZENT at DailyMed |
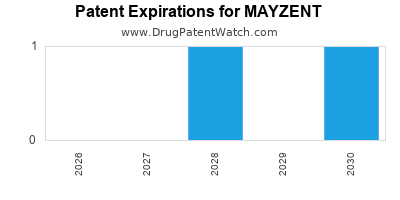

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for MAYZENT
Generic Entry Date for MAYZENT*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for MAYZENT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Robert Zivadinov, MD, PhD | Phase 4 |
| Novartis Pharmaceuticals | Phase 4 |
| Novartis Pharmaceuticals | Phase 3 |
Pharmacology for MAYZENT
| Drug Class | Sphingosine 1-phosphate Receptor Modulator |
| Mechanism of Action | Sphingosine 1-Phosphate Receptor Modulators |
Anatomical Therapeutic Chemical (ATC) Classes for MAYZENT
US Patents and Regulatory Information for MAYZENT
MAYZENT is protected by two US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of MAYZENT is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting MAYZENT
Immunosuppresant compounds and compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dosage regimen of an S1P receptor agonist
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATING MULTIPLE SCLEROSIS BY ADMINISTERING SIPONIMOD USING A TITRATION SCHEME TO REACH A MAINTENANCE DOSE
FDA Regulatory Exclusivity protecting MAYZENT
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
REVISIONS TO THE LABELING TO INCLUDE RESULTS FROM CLINICAL STUDY CBAF312A2130
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-001 | Mar 26, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-003 | Aug 24, 2021 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-001 | Mar 26, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-002 | Mar 26, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-003 | Aug 24, 2021 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-001 | Mar 26, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Novartis | MAYZENT | siponimod | TABLET;ORAL | 209884-001 | Mar 26, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for MAYZENT
See the table below for patents covering MAYZENT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2011224085 | Immunosuppressant compounds and compositions | ⤷ Try a Trial |
| Cyprus | 1112505 | ⤷ Try a Trial | |
| Canada | 2524047 | COMPOSES IMMUNOSUPPRESSEURS ET COMPOSITIONS (IMMUNOSUPPRESSANT COMPOUNDS AND COMPOSITIONS) | ⤷ Try a Trial |
| Japan | 2006528698 | ⤷ Try a Trial | |
| European Patent Office | 1633336 | COMPOSES IMMUNOSUPPRESSEURS ET COMPOSITIONS (IMMUNOSUPPRESSANT COMPOUNDS AND COMPOSITIONS) | ⤷ Try a Trial |
| Israel | 294658 | משטר מינון של אגוניסט לרצפטור s1p (Dosage regimen of an s1p receptor agonist) | ⤷ Try a Trial |
| Tunisia | 2011000281 | DOSAGE REGIMEN OF AN S1P RECEPTOR AGONIST | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MAYZENT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2379069 | C20200016 00335 | Estonia | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOOD;REG NO/DATE: EU/1/19/1414 15.01.2020 |
| 2379069 | 2020/022 | Ireland | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; REGISTRATION NO/DATE: EU/1/19/1414 20200113 |
| 2379069 | 21/2020 | Austria | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; REGISTRATION NO/DATE: EU/1/19/1414 (MITTEILUNG) 20200115 |
| 2379069 | SPC/GB20/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; REGISTERED: UK EU/1/19/1414 (NI) 20200115; UK PLGB00101/1189 20200115; UK PLGB00101/1190 20200115 |
| 2379069 | 2090022-1 | Sweden | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; REG. NO/DATE: EU/1/19/1414 20200115 |
| 2379069 | 2020C/526 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; AUTHORISATION NUMBER AND DATE: EU/1/19/1414 20200115 |
| 2379069 | CR 2020 00026 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SIPONIMOD; REG. NO/DATE: EU/1/19/1414 20200115 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


