LATUDA Drug Patent Profile
✉ Email this page to a colleague
When do Latuda patents expire, and what generic alternatives are available?
Latuda is a drug marketed by Sunovion Pharms Inc and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-nine patent family members in twenty-four countries.
The generic ingredient in LATUDA is lurasidone hydrochloride. There are twenty-six drug master file entries for this compound. Twenty-five suppliers are listed for this compound. Additional details are available on the lurasidone hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Latuda
A generic version of LATUDA was approved as lurasidone hydrochloride by ACCORD HLTHCARE on January 3rd, 2019.
Summary for LATUDA
| International Patents: | 69 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 104 |
| Clinical Trials: | 24 |
| Patent Applications: | 64 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for LATUDA |
| Drug Sales Revenues: | Drug sales revenues for LATUDA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LATUDA |
| What excipients (inactive ingredients) are in LATUDA? | LATUDA excipients list |
| DailyMed Link: | LATUDA at DailyMed |
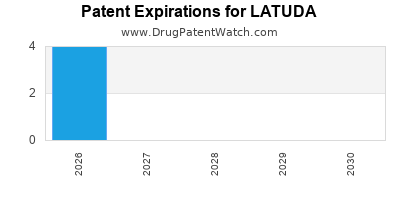
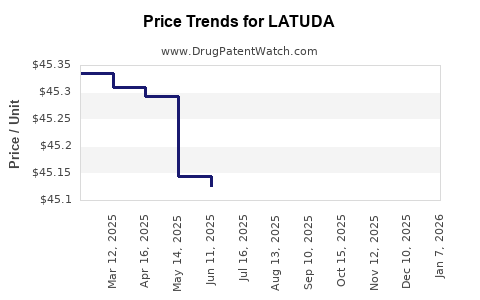
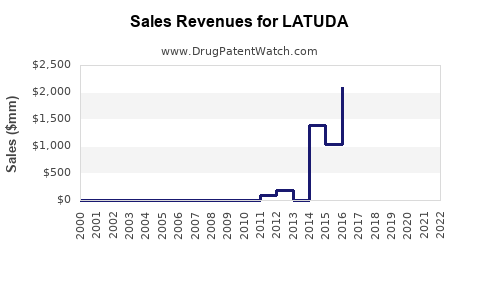
Recent Clinical Trials for LATUDA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Columbia University | Phase 4 |
| New York State Psychiatric Institute | Phase 4 |
| Astellas Pharma Global Development, Inc. | Phase 2 |
Pharmacology for LATUDA
| Drug Class | Atypical Antipsychotic |
Anatomical Therapeutic Chemical (ATC) Classes for LATUDA
Paragraph IV (Patent) Challenges for LATUDA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LATUDA | Tablets | lurasidone hydrochloride | 20 mg, 40 mg, 60 mg, 80 mg, and 120 mg | 200603 | 14 | 2014-10-28 |
US Patents and Regulatory Information for LATUDA
LATUDA is protected by eleven US patents.
Patents protecting LATUDA
Pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Remedy for integration dysfunction syndrome
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method of treatment for mental disorders
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SCHIZOPHRENIA
Agent for treatment of schizophrenia
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF MAJOR DEPRESSIVE EPISODES ASSOCIATED WITH BIPOLAR I DISORDER
Agent for treatment of schizophrenia
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SCHIZOPHRENIA
Method of treatment for mental disorders
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SCHIZOPHRENIA WITH IMPROVEMENT IN ATTENTION FUNCTION IN SCHIZOPHRENIA
Method of treatment for mental disorders
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF BIPOLAR DEPRESSION WITH IMPROVEMENT IN ATTENTION FUNCTION IN BIPOLAR DISORDER
Pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Process for producing imide compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-002 | Oct 28, 2010 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-005 | Jul 12, 2013 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-004 | Apr 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-004 | Apr 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-004 | Apr 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LATUDA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-001 | Oct 28, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-003 | Dec 7, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-005 | Jul 12, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-004 | Apr 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Sunovion Pharms Inc | LATUDA | lurasidone hydrochloride | TABLET;ORAL | 200603-001 | Oct 28, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for LATUDA
When does loss-of-exclusivity occur for LATUDA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06250340
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0611409
Estimated Expiration: ⤷ Sign Up
Patent: 2020005056
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 06510
Estimated Expiration: ⤷ Sign Up
China
Patent: 1184489
Estimated Expiration: ⤷ Sign Up
Patent: 2048734
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 14118
Estimated Expiration: ⤷ Sign Up
Patent: 14039
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 84242
Estimated Expiration: ⤷ Sign Up
Patent: 22783
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 84242
Estimated Expiration: ⤷ Sign Up
Patent: 22783
Estimated Expiration: ⤷ Sign Up
France
Patent: C0069
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 08379
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 400051
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 2006126681
Estimated Expiration: ⤷ Sign Up
Patent: 33120
Estimated Expiration: ⤷ Sign Up
Patent: 85105
Estimated Expiration: ⤷ Sign Up
Patent: 11126915
Estimated Expiration: ⤷ Sign Up
Luxembourg
Patent: 550
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 07014872
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 0690
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 84242
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 84242
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 98586
Estimated Expiration: ⤷ Sign Up
Patent: 07148997
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 84242
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1380088
Estimated Expiration: ⤷ Sign Up
Patent: 1552033
Estimated Expiration: ⤷ Sign Up
Patent: 080012306
Estimated Expiration: ⤷ Sign Up
Patent: 130122019
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 08687
Estimated Expiration: ⤷ Sign Up
Patent: 35478
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 59020
Estimated Expiration: ⤷ Sign Up
Patent: 0800197
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering LATUDA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 2422783 | ⤷ Sign Up | |
| Spain | 2408687 | ⤷ Sign Up | |
| Germany | 60327634 | ⤷ Sign Up | |
| Australia | 2004259305 | Process for producing imide compound | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2005009999 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LATUDA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1884242 | 2014/051 | Ireland | ⤷ Sign Up | PRODUCT NAME: LURASIDONE, PARTICULARLY A PHARMACEUTICALLY ACCEPTABLE SALT FORM AND ESPECIALLY THE HYDROCHLORIDE SALT THEREOF; REGISTRATION NO/DATE: EU/1/14/913/001-021 20140321 |
| 1884242 | 251 5024-2014 | Slovakia | ⤷ Sign Up | PRODUCT NAME: LURASIDON; REGISTRATION NO/DATE: EU/14/913/001 - EU/14/913/021 20140327 |
| 1884242 | C 2014 038 | Romania | ⤷ Sign Up | PRODUCT NAME: LURASIDONA, OPTIONAL SUB FORMA BAZEI EI LIBERE SAU CA SARURIACCEPTABILE FARMACEUTIC ALREA (EEA): EU/1/14/913; DATE OF FIRST AUTHORISATION IN EEA: 20140321 E ACESTEIA, IN SPECIAL CLORHIDRAT DE LURASIDONA -C28H36N4O2S; NATIONAL AUTHORISATION NUMBER: EU/1/14/913; DATE OF NATIONAL AUTHORISATION: 20140321; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC A |
| 1884242 | PA2014034 | Lithuania | ⤷ Sign Up | PRODUCT NAME: LURASIDONUM; REGISTRATION NO/DATE: EU/1/14/913 20140321 |
| 1884242 | SPC/GB14/063 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: LURASIDONE HYDROCHLORIDE; REGISTERED: UK EU/1/14/913/001 20140327; UK EU/1/14/913/002 20140327; UK EU/1/14/913/003 20140327; UK EU/1/14/913/004 20140327; UK EU/1/14/913/005 20140327; UK EU/1/14/913/006 20140327; UK EU/1/14/913/007 20140327; UK EU/1/14/913/008 20140327; UK EU/1/14/913/009 20140327; UK EU/1/14/913/010 20140327; UK EU/1/14/913/011 20140327; UK EU/1/14/913/012 20140327; UK EU/1/14/913/013 20140327; UK EU/1/14/913/014 20140327; UK EU/1/14/913/015 20140327; UK EU/1/14/913/016 20140327; UK EU/1/14/913/017 20140327; UK EU/1/14/913/018 20140327; UK EU/1/14/913/019 20140327; UK EU/1/14/913/020 20140327; UK EU/1/14/913/021 20140327 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


