JULUCA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Juluca, and when can generic versions of Juluca launch?
Juluca is a drug marketed by Viiv Hlthcare and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has four hundred and eighty patent family members in fifty-seven countries.
The generic ingredient in JULUCA is dolutegravir sodium; rilpivirine hydrochloride. There are seventeen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the dolutegravir sodium; rilpivirine hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Juluca
Juluca was eligible for patent challenges on August 12, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 24, 2031. This may change due to patent challenges or generic licensing.
There have been twenty-three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for JULUCA?
- What are the global sales for JULUCA?
- What is Average Wholesale Price for JULUCA?
Summary for JULUCA
| International Patents: | 480 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 3 |
| Formulation / Manufacturing: | see details |
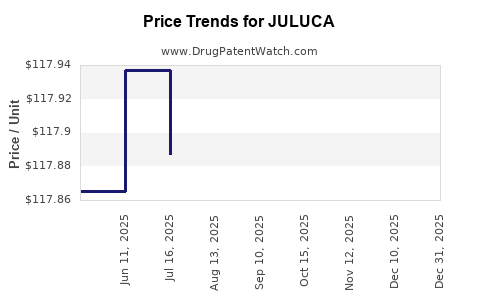
| Drug Prices: | Drug price information for JULUCA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for JULUCA |
| What excipients (inactive ingredients) are in JULUCA? | JULUCA excipients list |
| DailyMed Link: | JULUCA at DailyMed |
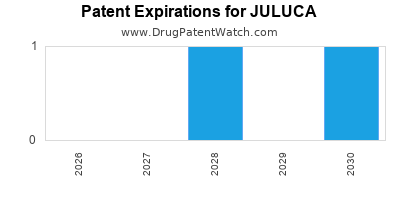
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for JULUCA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for JULUCA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| ViiV Healthcare | Phase 1/Phase 2 |
| Janssen Research & Development, LLC | Phase 1/Phase 2 |
| Indiana University | Phase 4 |
Pharmacology for JULUCA
Paragraph IV (Patent) Challenges for JULUCA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| JULUCA | Tablets | dolutegravir sodium; rilpivirine hydrochloride | 50 mg/25 mg | 210192 | 1 | 2019-11-19 |
US Patents and Regulatory Information for JULUCA
JULUCA is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of JULUCA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting JULUCA
Antiviral therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION
HIV inhibiting pyrimidines derivatives
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION
Substituted 5-hydroxy-3,4,6,9,9a, 10-hexanhydro-2h-1-oxa04a,8a-diaza-anthracene-6,10-dioness
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Synthesis of carbamoylpyridone HIV integrase inhibitors and intermediates
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for JULUCA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for JULUCA
When does loss-of-exclusivity occur for JULUCA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 51
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 11209788
Patent: Antiviral therapy
Estimated Expiration: ⤷ Sign Up
Patent: 14202404
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 14202405
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 14202406
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 16204987
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 17268621
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2012018670
Patent: combinação, uso de um composto, composição farmacêutica, e, pacote de paciente
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 87691
Patent: THERAPIE ANTIVIRALE (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 67453
Patent: COMBINAISONS A UTILISER POUR L'INHIBITION DE VIH-1 (COMBINATIONS FOR USE IN THE INHIBITION OF HIV-1)
Estimated Expiration: ⤷ Sign Up
Patent: 03988
Patent: COMBINAISONS A UTILISER POUR L'INHIBITION DU VIH-1 (COMBINATIONS FOR USE IN THE INHIBITION OF HIV-1)
Estimated Expiration: ⤷ Sign Up
Patent: 60290
Patent: COMBINAISONS A UTILISER POUR L'INHIBITION DU VIH-1 (COMBINATIONS FOR USE IN THE INHIBITION OF HIV-1)
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 12002080
Patent: Una combinación que comprende (4r,12as)-n-[2,4-fluoro-fenil)-metil]-3,4,6,8,12,12a-hexahidro-7-hidroxi-4-metil-6,8-dioxo-2h-pirido-{1',2':4,5]-pirazino-[2,1-b][1,3]-oxazin-9-carboxamida (gsk1349572) y uno o mas del grupo: abacavir, efavirenz y lopinavir, adicionalmente puede contener lamivudina, útil para el tratamiento del vih.
Estimated Expiration: ⤷ Sign Up
China
Patent: 2791129
Patent: Antiviral therapy
Estimated Expiration: ⤷ Sign Up
Patent: 5311033
Patent: Antiviral therapy
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 02152
Patent: En respuesta al requerimiento de pago de reivindicaciones adicionales a las 10 primeras,presenta nuevo capítulo reivindicatorio y paga.
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 120423
Patent: Terapia Antiviral
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0150770
Estimated Expiration: ⤷ Sign Up
Patent: 0180855
Estimated Expiration: ⤷ Sign Up
Patent: 0181531
Estimated Expiration: ⤷ Sign Up
Patent: 0240168
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 16509
Estimated Expiration: ⤷ Sign Up
Patent: 20457
Estimated Expiration: ⤷ Sign Up
Patent: 21040
Estimated Expiration: ⤷ Sign Up
Patent: 18029
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 31027
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Estimated Expiration: ⤷ Sign Up
Patent: 94972
Estimated Expiration: ⤷ Sign Up
Dominican Republic
Patent: 012000205
Patent: TERAPIA ANTIVIRAL
Estimated Expiration: ⤷ Sign Up
Patent: 021000147
Patent: COMBINACION TERAPEUTICA QUE COMPRENDE LAMIVUDINA
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 12012106
Patent: TERAPIA ANTIVIRAL
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 5176
Patent: КОМБИНАЦИЯ ДЛЯ ЛЕЧЕНИЯ ВИЧ-ИНФЕКЦИИ (COMBINATION FOR TREATING HIV INFECTION)
Estimated Expiration: ⤷ Sign Up
Patent: 2868
Patent: КОМБИНАЦИЯ ДЛЯ ЛЕЧЕНИЯ ВИЧ-ИНФЕКЦИИ (COMBINATION FOR TREATING HIV INFECTION)
Estimated Expiration: ⤷ Sign Up
Patent: 7601
Patent: КОМБИНАЦИЯ ДЛЯ ЛЕЧЕНИЯ ВИЧ-ИНФЕКЦИИ (COMBINATION FOR TREATING HIV INFECTION)
Estimated Expiration: ⤷ Sign Up
Patent: 1290583
Patent: ПРОТИВОВИРУСНАЯ ТЕРАПИЯ
Estimated Expiration: ⤷ Sign Up
Patent: 1690872
Patent: ПРОТИВОВИРУСНАЯ ТЕРАПИЯ
Estimated Expiration: ⤷ Sign Up
Patent: 1892277
Patent: ПРОТИВОВИРУСНАЯ ТЕРАПИЯ
Estimated Expiration: ⤷ Sign Up
Patent: 2190473
Patent: ПРОТИВОВИРУСНАЯ ТЕРАПИЯ
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 31027
Patent: Combinaison thérapeutique comprenant du dolutégravir, de l'abacavir et de la lamivudine (Therapeutic combination comprising dolutegravir, abacavir and lamivudine)
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Patent: THÉRAPIE ANTIVIRALE (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Patent: THERAPIE ANTIVIRALE (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 51249
Patent: THÉRAPIE ANTIVIRALE (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 94972
Patent: ZUSAMMENSETZUNGEN AUS DOLUTEGRAVIR UND LAMIVUDIN ZUR BEHANDLUNG VON DURCH HIV VERURSACHTEN INFEKTIONEN (COMBINATIONS OF DOLUTEGRAVIR AND LAMIVUDINE FOR THE TREAETMENT OF HIV INFECTION)
Estimated Expiration: ⤷ Sign Up
Patent: 16599
Patent: THERAPIE ANTIVIRALE (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 94972
Estimated Expiration: ⤷ Sign Up
France
Patent: C1043
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 79522
Patent: 包含 之治療組合 (THERAPEUTIC COMBINATION COMPRISING DOLUTEGRAVIR, ABACAVIR AND LAMIVUDINE DOLUTEGRAVIRABACVIR LAMIVUDINE)
Estimated Expiration: ⤷ Sign Up
Patent: 09629
Patent: 抗病毒治療 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 50335
Patent: 抗病毒治療 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 26849
Estimated Expiration: ⤷ Sign Up
Patent: 37812
Estimated Expiration: ⤷ Sign Up
Patent: 40554
Estimated Expiration: ⤷ Sign Up
Patent: 800042
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 1007
Patent: שילובים רוקחיים לטיפול אנטי וירלית (Pharmaceutical combinations for antiviral therapy)
Estimated Expiration: ⤷ Sign Up
Patent: 5182
Patent: שילוב של תרכובות לטיפול אנטי ויראלי (Combination of compounds for antiviral therapy)
Estimated Expiration: ⤷ Sign Up
Patent: 7267
Patent: שילוב של תרכובות לטיפול אנטי ויראלי (Combination of compounds for antiviral therapy)
Estimated Expiration: ⤷ Sign Up
Patent: 7658
Patent: שילוב של תרכובות לטיפול אנטי ויראלי (Combination of compounds for antiviral therapy)
Estimated Expiration: ⤷ Sign Up
Patent: 1959
Patent: שילוב תרכובות לריפוי אנטי ויראלי (Combination of compounds for antiviral therapy)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 68386
Estimated Expiration: ⤷ Sign Up
Patent: 13518107
Estimated Expiration: ⤷ Sign Up
Patent: 16145204
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 17008087
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 18127473
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 19167371
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 21091705
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 22071126
Patent: 抗ウイルス療法
Estimated Expiration: ⤷ Sign Up
Patent: 23085431
Patent: 抗ウイルス療法 (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 932970
Estimated Expiration: ⤷ Sign Up
Patent: 2018013
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Estimated Expiration: ⤷ Sign Up
Patent: 94972
Estimated Expiration: ⤷ Sign Up
Luxembourg
Patent: 0090
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 8334
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 6891
Patent: COMBINACIONES DE COMPUESTOS QUE COMPRENDEN INHIBIDORES DE LA INTEGRASA DE VIH Y OTROS AGENTES TERAPÉUTICOS. (ANTIVIRAL THERAPY.)
Estimated Expiration: ⤷ Sign Up
Patent: 7937
Patent: COMBINACIONES DE COMPUESTOS QUE COMPRENDEN INHIBIDORES DE LA INTEGRASA DE VIH Y OTROS AGENTES TERAPÉUTICOS. (ANTIVIRAL THERAPY.)
Estimated Expiration: ⤷ Sign Up
Patent: 7938
Patent: COMBINACIONES DE COMPUESTOS QUE COMPRENDEN INHIBIDORES DE LA INTEGRASA DE VIH Y OTROS AGENTES TERAPÉUTICOS. (ANTIVIRAL THERAPY.)
Estimated Expiration: ⤷ Sign Up
Patent: 12008774
Patent: TERAPIA ANTIVIRAL. (ANTIVIRAL THERAPY.)
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 182
Patent: TERAPEUTSKA KOMBINACIJA KOJA SADRŽI DOLUTEGRAVIR, ABAKAVIR l LAMIVUDIN (THERAPEUTIC COMBINATION COMPRISING DOLUTEGRAVIR, ABACAVIR AND LAMIVUDINE)
Estimated Expiration: ⤷ Sign Up
Patent: 058
Patent: ANTIVIRUSNA TERAPIJA (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 002
Patent: Thérapie antivirale
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 1319
Patent: Antiviral combinations involving (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido[1’,2’:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
Estimated Expiration: ⤷ Sign Up
Patent: 7824
Patent: Antiviral combinations involving (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2hpyrido[1’,2’:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
Estimated Expiration: ⤷ Sign Up
Patent: 7826
Patent: Antiviral combinations involving (3s,11ar)-n-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
Estimated Expiration: ⤷ Sign Up
Patent: 7827
Patent: Antiviral combinations involving (3s, 11ar)-n-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 18036
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 121524
Patent: COMBINACIONES DE COMPUESTOS QUE COMPRENDEN INHIBIDORES DE LA INTEGRASA DE VIH Y OTROS AGENTES TERAPEUTICOS
Estimated Expiration: ⤷ Sign Up
Patent: 160180
Patent: COMBINACIONES DE COMPUESTOS QUE COMPRENDEN INHIBIDORES DE LA INTEGRASA DE VIH Y OTROS AGENTES TERAPEUTICOS.
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 016500195
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 018502489
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 31027
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 31027
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Estimated Expiration: ⤷ Sign Up
Patent: 94972
Estimated Expiration: ⤷ Sign Up
San Marino
Patent: 01500177
Patent: Combinazione terapeutica comprendente dolutegravir, abacavir e lamivudina
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 123
Patent: TERAPEUTSKA KOMBINACIJA KOJA SADRŽI DOLUTEGRAVIR, ABACAVIR I LAMIVUDINE (THERAPEUTIC COMBINATION COMPRISING DOLUTEGRAVIR, ABACAVIR AND LAMIVUDINE)
Estimated Expiration: ⤷ Sign Up
Patent: 323
Patent: ANTIVIRUSNA TERAPIJA (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 728
Patent: ANTIVIRUSNA TERAPIJA (ANTIVIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 183
Patent: KOMBINACIJE DOLUTEGRAVIRA I LANIVUDINA ZA TRETMAN HIV INFEKCIJE (COMBINATIONS OF DOLUTEGRAVIR AND LAMIVUDINE FOR THE TREATMENT OF HIV INFECTION)
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 2614
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 201509476R
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 201707183T
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 31027
Estimated Expiration: ⤷ Sign Up
Patent: 32970
Estimated Expiration: ⤷ Sign Up
Patent: 27542
Estimated Expiration: ⤷ Sign Up
Patent: 94972
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1205586
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1830715
Estimated Expiration: ⤷ Sign Up
Patent: 1883750
Estimated Expiration: ⤷ Sign Up
Patent: 1964923
Estimated Expiration: ⤷ Sign Up
Patent: 120128640
Patent: ANTIBIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Patent: 160111536
Patent: 항바이러스 치료 (ANTIBIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 170078868
Patent: 항바이러스 치료 (ANTIBIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Patent: 180078358
Patent: 항바이러스 치료 (ANTIBIRAL THERAPY)
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 43066
Estimated Expiration: ⤷ Sign Up
Patent: 70811
Estimated Expiration: ⤷ Sign Up
Patent: 88925
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 12000376
Patent: ANTIVIRAL THERAPY
Estimated Expiration: ⤷ Sign Up
Turkey
Patent: 1807704
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 5556
Patent: КОМБИНАЦИЯ СОЕДИНЕНИЙ, СОДЕРЖАЩАЯ ИНГИБИТОРЫ ВИЧ ИНТЕГРАЗЫ С ДРУГИМИ ТЕРАПЕВТИЧЕСКИМИ АГЕНТАМИ;КОМБІНАЦІЯ СПОЛУК, ЩО МІСТИТЬ ІНГІБІТОРИ ВІЛ ІНТЕГРАЗИ З ІНШИМИ ТЕРАПЕВТИЧНИМИ АГЕНТАМИ (COMBINATION OF COMPOUNDS COMPRISING HIV INTEGRASE INHIBITORS WITH OTHER THERAPEUTICAL AGENTS)
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering JULUCA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 200701826 | Furamate of 4-[[4-[[4-(2-cyanoethenyl)-2,6-dimethylphenyl] amino]-2-pyrimidinyl]amino]benzonitrile | ⤷ Sign Up |
| Spain | 2567197 | ⤷ Sign Up | |
| Mexico | 312216 | Polycyclic carbamoylpyridone derivative having HIV integrase inhibitory activity | ⤷ Sign Up |
| Serbia | 57323 | ANTIVIRUSNA TERAPIJA (ANTIVIRAL THERAPY) | ⤷ Sign Up |
| Russian Federation | 2638923 | Синтез карбамоилпиридоновых ингибиторов интегразы ВИЧ и промежуточных соединений (SYNTHESIS OF CARBAMOIL-PYRIDONE INHIBITORS OF HIV INTEGRASE AND INTERMEDIATE COMPOUNDS) | ⤷ Sign Up |
| Austria | 517891 | ⤷ Sign Up | |
| Croatia | P20040096 | HIV INHIBITING PYRIMIDINES DERIVATIVES | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for JULUCA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1632232 | PA2016042 | Lithuania | ⤷ Sign Up | PRODUCT NAME: RILPIVIRINO HIDROCHLORIDAS + EMTRICITABINAS + TENOFOVIRO ALAFENAMIDAS; REGISTRATION NO/DATE: EU/1/16/1112 20160621 |
| 2465580 | 132021000000098 | Italy | ⤷ Sign Up | PRODUCT NAME: CABOTEGRAVIR(VOCABRIA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1481, 20201221 |
| 1419152 | C 2012 016 | Romania | ⤷ Sign Up | PRODUCT NAME: COMBINATIE DE RILPIVIRINA SI TOATE FORMELE ECHIVALENTETERAPEUTIC ALE ACESTEIA CUM AR FI SARURILE DE ADITIE ALE RILPIVIRINEI ACCEPTABILE FARMACEUTIC, INCLUSIV SAREARILPIVIRINEI CU ACIDUL CLORHIDRIC, SI TENOFOVIR, IN PARTICULAR FUMARAT DE TENOFOVIRDISOPROXIL; NATIONAL AUTHORISATION NUMBER: EU/1/11/737/001, EU/1/11/737/002; DATE OF NATIONAL AUTHORISATION: 20111128; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/11/737/001, EU/1/11/737/002; DATE OF FIRST AUTHORISATION IN EEA: 20111128 |
| 1874117 | C201430032 | Spain | ⤷ Sign Up | PRODUCT NAME: DOLUTEGRAVIR O UNA SAL O SOLVATO DEL MISMO FARMACEUTICAMENTE ACEPTABLE, INCLUIDA LA SAL SODICA DE DOLUTEGRAVIR.; NATIONAL AUTHORISATION NUMBER: EU/1/13/892; DATE OF AUTHORISATION: 20140116; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/892; DATE OF FIRST AUTHORISATION IN EEA: 20140116 |
| 1419152 | C20120012 00058 | Estonia | ⤷ Sign Up | PRODUCT NAME: EVIPLERA - EMTRITSITABIIN/RILPIVIRIIN (HUEDRO-;REG NO/DATE: C(2011)8956 FINAL 28.11.2011 |
| 1874117 | 122014000066 | Germany | ⤷ Sign Up | PRODUCT NAME: DOLUTEGRAVIR ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ ODER SOLVAT DAVON, EINSCHLIESSLICH DOLUTEGRAVIRNATRIUM; REGISTRATION NO/DATE: EU/1/13/892/001-002 20140116 |
| 1874117 | 517 | Finland | ⤷ Sign Up | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


