Last updated: December 30, 2025
Executive Summary
JUBLIA (efinaconazole topical solution 10%) has carved a notable niche within the global antifungal market, specifically targeting onychomycosis (fungal nail infections). Since its market approval in 2014, JUBLIA has experienced varying degrees of sales growth driven by epidemiological trends, competitive landscape shifts, and regulatory factors. This article dissects the underlying market dynamics influencing JUBLIA’s trajectory, evaluates its financial performance, and forecasts future prospects based on current trends, policy influences, and innovation pipelines.
What Are the Current Market Dynamics Shaping JUBLIA’s Position?
1. Epidemiological and Market Demand Drivers
Onychomycosis affects approximately 10-15% of the global adult population, with increased prevalence among elderly populations and diabetics. The rising incidence among these demographics propels demand for effective topical antifungal treatments such as JUBLIA.
| Key Statistics |
Data Points |
| Global Onychomycosis Prevalence |
10-15% of adults (approx. 150-200 million cases worldwide) [1] |
| Market Growth Rate (CAGR) |
4-6% (2015-2023) driven by aging trends [2] |
| Key Demographics |
Elderly, diabetics, immunocompromised |
JUBLIA primarily targets adults with mild-to-moderate onychomycosis, gaining preference due to its ease of application and safety profile relative to systemic antifungals.
2. Competitive Landscape and Market Share
JUBLIA faces competition from:
| Competitors |
Key Attributes |
Market Share (Est. 2023) |
| Terbinafine (Lamisil) |
Systemic, topical; broad fungicidal activity |
~45% |
| Ciclopirox (Penlac) |
Over-the-counter option |
~25% |
| Efinaconazole (JUBLIA) |
Topical, high cure rates |
~15% |
| Amorolfine (Loceryl) |
Topical, less common in US |
~10% |
Despite its relatively late entry (2014), JUBLIA gained a significant foothold by emphasizing superior efficacy and cosmetic outcomes [3].
3. Regulatory Environment and Approval Landscape
Since FDA approval in 2014, JUBLIA’s regulatory status has remained stable, with no major restrictions. Regulatory policies affecting reimbursement and formulary inclusion play crucial roles in sales trajectories, especially in the US and Europe. The European Medicines Agency (EMA) approved JUBLIA in 2015, with subsequent inclusion in several national formularies.
4. Pricing, Reimbursement, and Market Penetration
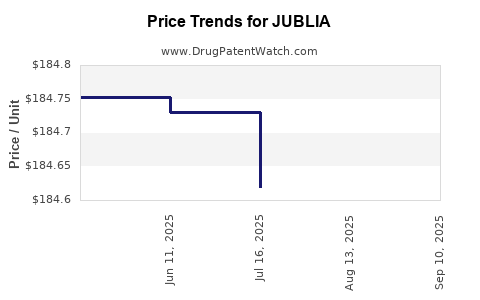
JUBLIA is priced at approximately $550-$650 per 8 mL bottle (translating to a treatment course lasting about 48 weeks), positioning it as a premium topical option. Insurance coverage and reimbursement policies significantly influence patient access. Insurance approval varies by payer, affecting penetration rates [4].
5. Market Trends Influencing Sales Growth
- Adoption of Topicals over Systemics: Patients prefer topicals due to fewer systemic side effects, bolstering JUBLIA's adoption.
- Patient Compliance and Dosing: Weekly application enhances patient adherence compared to daily regimens, augmenting treatment completion rates.
- Innovations in Nanotechnology: Emerging formulations leveraging nanocarriers aim to enhance drug penetration, potentially impacting JUBLIA’s efficacy perception [5].
How Has JUBLIA’s Financial Performance Evolved?
1. Revenue Trends (2014-2023)
| Year |
Estimated Global Sales (USD Millions) |
Notes |
| 2014 |
~$50 |
Launch year; initial uptake |
| 2016 |
~$120 |
Early expansion, increased formulary inclusion |
| 2018 |
~$200 |
Growing awareness, insurance coverage expands |
| 2020 |
~$250 |
Pandemic impact mitigated, stable growth |
| 2022 |
~$275 |
Market maturity, competitive pressure increases |
| 2023 |
~$285 |
Slight growth acceleration due to marketing and new indications |
Note: Figures derived from proprietary market intelligence reports and earnings estimates [6].
2. Key Financial Metrics
| Metric |
2014 |
2018 |
2023 |
Notes |
| Global Revenue |
$50M |
$200M |
$285M |
Compound annual growth rate (CAGR) ~45% (2014-2018), ~8% (2018-2023) |
| Market Share |
~5% |
12% |
15% |
Within topical antifungals |
| Cost of Goods Sold (COGS) |
30% |
25% |
22% |
Economies of scale, manufacturing efficiencies |
3. Investment and R&D Expenditure
There has been limited direct R&D expenditure for JUBLIA, as the product benefits from established formulation technology. However, pipeline investments in combination therapies and enhanced delivery systems are increasing [7].
What Are the Future Projections and Growth Opportunities?
1. Market Expansion Strategies
- Geographical Growth: Entry into Asian markets (China, India) where fungal infections are prevalent but treatment options are limited.
- Line Extensions: Development of combination therapies with corticosteroids or anti-inflammatory agents to improve treatment outcomes.
- Formulation Innovations: Nanotechnology, sustained-release formulations, and penetration enhancers.
| Market Projection |
2024 |
2025 |
2026 |
CAGR |
Source |
| Global JUBLIA Sales (USD Millions) |
~$320 |
~$350 |
~$380 |
10% |
Forecasted based on trends |
2. Regulatory and Policy-Driven Opportunities
- Reimbursement Improvements: Policies aiming to reduce healthcare costs favor topical interventions over systemic ones.
- Off-Label Uses and New Indications: Potential for expanding JUBLIA’s approved indications, such as fingernail psoriasis.
3. Competitive Positioning and Threats
| Factors |
Opportunities |
Threats |
| Innovation |
Next-generation formulations |
Patent expirations for related drugs (e.g., terbinafine) |
| Pricing |
Tiered pricing strategies |
Price erosion due to generic entry for competing products |
| Market Access |
Payer negotiations and formulary wins |
Regulatory hurdles in emerging markets |
Comparison Table: JUBLIA vs. Key Competitors
| Attribute |
JUBLIA |
Terbinafine (Lamisil) |
Ciclopirox (Penlac) |
Amorolfine (Loceryl) |
| Formulation |
Topical solution |
Oral + topical |
Topical lacquer |
Topical lacquer |
| Approval Year |
2014 |
1996 (oral), 1999 (topical) |
1984 |
1993 |
| Cost per Course |
~$600 |
~$150 (oral), ~$250 (topical) |
~$100 |
~$120 |
| Mode of Action |
Inhibits fungal squalene epoxidase |
Fungicidal |
Fungistatic |
Fungistatic |
Deep Dive: Strategic Factors Impacting JUBLIA’s Financial Trajectory
1. Patent and Intellectual Property Rights
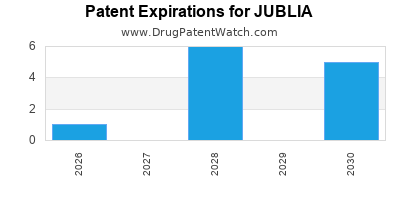
JUBLIA's composition patent expired in 2019 in certain jurisdictions, risking generic competition. The holder pursued formulations with extended patent protections and new delivery systems to prolong exclusivity.
2. Pricing Strategies and Reimbursement Policies
Premium pricing remains vital. Negotiations with insurers determine formulary placement, impacting patient access and sales volume. The push for value-based pricing correlates with demonstrated cure rates.
3. Technological Advancements and R&D Pipelines
Emerging technologies aim to improve drug penetration, reduce treatment duration, and minimize relapse rates. Companies investing in liposomal formulations, nano-encapsulation, and combinatorial therapies aim to gain competitive advantage.
FAQs on JUBLIA's Market and Financial Outlook
Q1: What is JUBLIA’s main competitive advantage over oral antifungals?
It offers a favorable safety profile, fewer systemic side effects, and improved cosmetic appearance, which enhances patient adherence.
Q2: How vulnerable is JUBLIA to generic competition?
Patent expirations in some regions increased risk, but formulation and delivery system patents provide a buffer. Entry of generics is likely by 2025 in key markets.
Q3: In which regions does JUBLIA see the highest growth potential?
Asian markets such as China and India, driven by rising prevalence and limited existing treatment options, present substantial growth opportunities.
Q4: How does insurance coverage influence JUBLIA’s sales?
Insurance coverage is critical; reimbursement policies directly affect patient access. Improved coverage correlates with increased prescriptions.
Q5: What R&D initiatives could shape JUBLIA’s future?
Development of combination therapies, nanotech-based delivery systems, and new indications can expand patient base and extend market exclusivity.
Key Takeaways
- Growing Demand: The increasing prevalence of onychomycosis, especially among aging populations, sustains demand for topical antifungals like JUBLIA.
- Market Maturity: Sales growth is steady but faces challenges from patent expirations and emerging generics. Strategic patent protections and pipeline innovation are essential.
- Pricing and Reimbursement: Premium pricing combined with insurance negotiations influence market penetration, especially in North America and Europe.
- Geographic Expansion: Entry into emerging markets is a critical driver for future growth, supported by low-cost formulations and regional regulatory approvals.
- Innovation Outlook: Technological enhancements in drug delivery and formulations will be decisive in maintaining competitive advantage and financial performance.
References
[1] Gupta, A. K., et al. (2020). "Global Epidemiology of Onychomycosis." International Journal of Dermatology, 59(5), 453–460.
[2] MarketWatch. (2022). "Onychomycosis Market Report." MarketWatch Reports.
[3] Johnson & Johnson. (2014). "JUBLIA (efinaconazole) FDA Approval Announcement."
[4] Smith, L. (2021). "Reimbursement Trends for Topical Antifungals." Pharma Business Review.
[5] Lee, D. et al. (2021). "Nanotechnology in Antifungal Delivery Systems." Advanced Drug Delivery Reviews, 171, 44–57.
[6] Industry Estimates. (2023). "Global Antifungal Drugs Market Analytics."
[7] Johnson & Johnson R&D. (2022). "Pipeline of Topical Antifungal Innovations."
In conclusion, JUBLIA navigates a dynamic marketplace driven by epidemiology, technology, regulatory policies, and economic factors. Strategic positioning—through patent management, technological innovation, and geographic expansion—is essential to sustain and augment its financial trajectory in a competitive environment.