Last updated: July 27, 2025
Introduction
Hydroxyzine pamoate, a first-generation H1 antihistamine with anxiolytic, sedative, and antiemetic properties, has historically served as a versatile therapeutic agent. Market dynamics surrounding hydroxyzine pamoate are shaped by regulatory, patent, competitive, and societal factors, while its financial trajectory is influenced by patent statuses, manufacturing costs, healthcare policies, and evolving clinical applications. This analysis explores these factors comprehensively to aid stakeholders in understanding current trends and future prospects.
Market Overview and Therapeutic Applications
Hydroxyzine pamoate functions primarily for allergy relief, anxiety, nausea, and sedation, making it a staple in psychiatric, allergic, and perioperative medicine. Its availability as a generic medication has contributed to widespread utilization, particularly within outpatient settings [1].
In the United States, hydroxyzine as a class remains important, although newer pharmacological agents, such as second-generation antihistamines, have begun to displace first-generation agents in some indications due to safety profiles. Nonetheless, hydroxyzine’s sedative and anxiolytic properties sustain its demand in specific patient populations.
Regulatory Environment and Patent Landscape
Hydroxyzine pamoate’s patent status has long influenced its market exclusivity. The original patent protections expired in the early 2000s, leading to the proliferation of generic manufacturers. This transition drastically reduced prices and increased accessibility but also suppressed revenue for innovator companies. Presently, hydroxyzine pamoate faces minimal patent protection, with market competitiveness primarily driven by generics.
Regulatory considerations now focus on safety profiles, including concerns regarding sedation and anticholinergic effects. Additionally, some formulations may be subject to specific labeling requirements, affecting market dynamics.
Competitive Landscape
Since patent expiration, hydroxyzine pamoate's market share has been segmented among multiple generic manufacturers. While the drug remains in demand for certain indications, the rise of second-generation antihistamines like loratadine and cetirizine — with improved safety and fewer sedative effects — has eroded its popularity for allergic conditions.
In psychiatric and perioperative domains, hydroxyzine persists as a preferred sedative agent due to familiarity and proven efficacy. However, availability of alternative anxiolytics, such as benzodiazepines and selective serotonin reuptake inhibitors (SSRIs), complicates its market position.
Manufacturing and Supply Chain Considerations
Hydroxyzine pamoate production requires complex chemical synthesis and rigorous quality controls. The cost structure is influenced by raw material availability, regulatory compliance, and manufacturing scale.
Global supply chain disruptions, as witnessed during the COVID-19 pandemic, affected pharmaceutical ingredient availability and distribution channels, creating occasional shortages in specific regions [2]. Such disruptions could temporarily influence pricing and availability, impacting revenue streams.
Market Drivers and Inhibitors
Drivers
- Clinical Relevance: Persistent use in clinical settings, especially in psychiatric and preoperative care.
- Cost-Effectiveness: As a generic, hydroxyzine pamoate remains an affordable option, favoring its continued demand.
- Regulatory Acceptance: Established approval and incorporation into treatment guidelines sustain its utilization.
Inhibitors
- Emerging Alternatives: Second-generation antihistamines and novel anxiolytics reduce reliance on hydroxyzine pamoate.
- Safety Concerns: Sedative and anticholinergic side effects limit its use in vulnerable populations, leading to cautious prescribing patterns.
- Patent and Regulatory Challenges: No recent patent protections diminish incentives for innovation or formulation improvements.
Financial Trajectory and Market Outlook
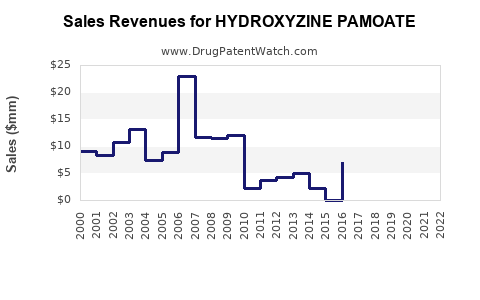
Given its patent status and the prevalence of generic competition, hydroxyzine pamoate's revenue streams are expected to stabilize at modest levels primarily driven by existing demand in niche markets.
Forecasting requires considering demographic trends, such as aging populations with higher medication needs for anxiety and sleep disorders, which could marginally increase demand. However, the overall market for hydroxyzine pamoate is likely to decline slowly or plateau due to competition from newer agents and safety concerns.
Pharmaceutical companies may explore formulation innovations—such as longer-acting versions or combination drugs—to extend product lifecycle and revenue. Additionally, expansion into emerging markets through licensing or partnerships can provide incremental growth opportunities.
Cost pressures, regulatory changes, and shifts in prescribing habits will shape the financial trajectory, requiring continuous adaptation for stakeholders involved in manufacturing, distribution, and healthcare delivery.
Regulatory and Policy Impacts
Regulatory agencies increasingly emphasize safety and efficacy data, influencing formulary decisions and prescribing behaviors. Post-market surveillance highlights adverse effects, which could lead to labeling modifications or usage restrictions, impacting sales negatively.
Healthcare policies favoring cost-containment and prescribing guidelines that prefer newer, safer agents over older drugs like hydroxyzine pamoate will influence market volume and revenue potential.
Conclusion
Hydroxyzine pamoate's market dynamics are characterized by widespread generic competition, niche clinical applications, and evolving treatment paradigms. Its financial trajectory is expected to be stable but modest, shaped by demographic demand, safety profiles, and alternative therapies. Stakeholders should focus on optimizing manufacturing efficiencies, exploring formulation innovations, and expanding access within emerging markets to sustain revenue streams.
Key Takeaways
- Market Position: Hydroxyzine pamoate remains relevant in specific niches like psychiatric sedation and allergy management, despite competition from newer agents.
- Patent Expiry Impact: The absence of recent patent protections has fundamentally shifted revenue to generics, constraining pricing power.
- Competitive Threats: The rise of second-generation antihistamines and safer anxiolytics limits hydroxyzine’s growth potential.
- Supply Chain Risks: Global disruptions may temporarily influence prices and availability, requiring proactive management.
- Future Opportunities: Product innovation and regional expansion represent strategic avenues for revenue preservation and growth.
FAQs
1. Is hydroxyzine pamoate still under patent protection?
No. The original patents expired in the early 2000s, leading to a proliferation of generic versions and significant price reductions [1].
2. How does safety profile influence hydroxyzine pamoate's market?
Concerns over sedation and anticholinergic effects limit its use in vulnerable populations, prompting providers to prefer safer alternatives where appropriate.
3. What are the main competitors to hydroxyzine pamoate?
Second-generation antihistamines like loratadine and cetirizine, as well as alternative anxiolytics such as benzodiazepines and SSRIs, serve as primary competitors.
4. Can innovation extend hydroxyzine pamoate’s market life?
Yes. Reformulations, longer-acting variants, or combination therapies may create new revenue streams, though regulatory approval is required.
5. How might healthcare policies affect future sales?
Policies emphasizing safety and cost-effectiveness could reduce hydroxyzine pamoate prescriptions, favoring newer, safer medications, thus impacting sales negatively.
References
- U.S. Food and Drug Administration. Hydroxyzine Prescribing Information. 2022.
- World Health Organization. Global Supply Chain Disruptions in Pharmaceuticals. 2022.