FIRMAGON Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Firmagon, and when can generic versions of Firmagon launch?
Firmagon is a drug marketed by Ferring and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and seven patent family members in twenty-four countries.
The generic ingredient in FIRMAGON is degarelix acetate. One supplier is listed for this compound. Additional details are available on the degarelix acetate profile page.
DrugPatentWatch® Generic Entry Outlook for Firmagon
Firmagon was eligible for patent challenges on December 24, 2012.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 10, 2029. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (degarelix acetate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for FIRMAGON?
- What are the global sales for FIRMAGON?
- What is Average Wholesale Price for FIRMAGON?
Summary for FIRMAGON
| International Patents: | 107 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 57 |
| Patent Applications: | 3,516 |
| Drug Prices: | Drug price information for FIRMAGON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for FIRMAGON |
| What excipients (inactive ingredients) are in FIRMAGON? | FIRMAGON excipients list |
| DailyMed Link: | FIRMAGON at DailyMed |
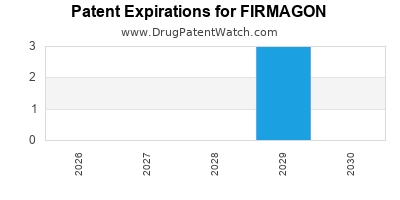

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for FIRMAGON
Generic Entry Date for FIRMAGON*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for FIRMAGON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Eli Lilly and Company | Phase 1/Phase 2 |
| Bayer | Phase 1/Phase 2 |
| Praful Ravi | Phase 1/Phase 2 |
Paragraph IV (Patent) Challenges for FIRMAGON
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FIRMAGON | Powder for Injection | degarelix acetate | 80 mg/vial and 120 mg/vial | 022201 | 1 | 2019-12-20 |
US Patents and Regulatory Information for FIRMAGON
FIRMAGON is protected by seven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of FIRMAGON is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for FIRMAGON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-002 | Dec 24, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for FIRMAGON
When does loss-of-exclusivity occur for FIRMAGON?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09213748
Patent: Method of treating prostate cancer with the GnRH antagonist degarelix
Estimated Expiration: ⤷ Get Started Free
Patent: 09213751
Patent: Treatment of metastatic stage prostate cancer with degarelix
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0908127
Patent: Método para o tratamento do câncer da próstata com degarelix antagonista do gnrh
Estimated Expiration: ⤷ Get Started Free
Patent: 0908129
Patent: Método de tratamento de câncer de próstata em estágio metastático
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 14444
Patent: PROCEDE DE TRAITEMENT DU CANCER DE LA PROSTATE PAR LE DEGARELIX, ANTAGONISTE DE GNRH (METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 14445
Patent: TRAITEMENT DU CANCER DE LA PROSTATE AU STADE METASTASIQUE PAR LE DEGARELIX (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1939020
Patent: Treatment of metastatic stage prostate cancer with degarelix
Estimated Expiration: ⤷ Get Started Free
Patent: 1998861
Patent: Method of treating prostate cancer with the gnrh antagonist degarelix
Estimated Expiration: ⤷ Get Started Free
Patent: 3990107
Patent: Method of treating prostate cancer with the GNRH antagonist degarelix
Estimated Expiration: ⤷ Get Started Free
Patent: 7412726
Patent: 用GNRH拮抗剂地加瑞克治疗前列腺癌的方法 (Method of treating prostate cancer with the GnRH antagonist degarelix)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0140665
Estimated Expiration: ⤷ Get Started Free
Patent: 0150290
Estimated Expiration: ⤷ Get Started Free
Patent: 0150633
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 15561
Estimated Expiration: ⤷ Get Started Free
Patent: 16289
Estimated Expiration: ⤷ Get Started Free
Patent: 16341
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 49859
Estimated Expiration: ⤷ Get Started Free
Patent: 05204
Estimated Expiration: ⤷ Get Started Free
Patent: 50012
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7582
Patent: ЛЕЧЕНИЕ МЕТАСТАТИЧЕСКОЙ СТАДИИ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ ДЕГАРЕЛИКСОМ (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 0543
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ (METHOD OF TREATING PROSTATE CANCER WITH THE GONADOTROPHIN RELEASING HORMONE (GnRH) ANTAGONIST DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 6521
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ (METHOD OF TREATING PROSTATE CANCER WITH GONADOTROPHIN RELEASING HORMONE (GnRH) ANTAGONIST DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 6695
Patent: ПРИМЕНЕНИЕ КОМПОЗИЦИИ, СОДЕРЖАЩЕЙ ДЕГАРЕЛИКС, ДЛЯ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ У СУБЪЕКТА, ИМЕЮЩЕГО ПОВЫШЕННЫЙ УРОВЕНЬ ХОЛЕСТЕРИНА (USE OF A COMPOSITION COMPRISING DEGARELIX IN TREATING PROSTATE CANCER IN A SUBJECT HAVING INCREASED CHOLESTEROL LEVEL)
Estimated Expiration: ⤷ Get Started Free
Patent: 0901074
Patent: ЛЕЧЕНИЕ МЕТАСТАТИЧЕСКОЙ СТАДИИ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
Patent: 0901075
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
Patent: 1300741
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
Patent: 1300742
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GNRH) ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 49858
Patent: PROCÉDÉ DE TRAITEMENT DU CANCER DE LA PROSTATE PAR LE DEGARELIX, ANTAGONISTE DE GNRH (METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 49859
Patent: TRAITEMENT DU CANCER DE LA PROSTATE AU STADE MÉTASTASIQUE PAR LE DEGARELIX (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 05204
Patent: Procédé de traitement du cancer de la prostate par l'antagoniste de GNRH degarelix (Method of treating prostate cancer with the GnRH antagonist degarelix)
Estimated Expiration: ⤷ Get Started Free
Patent: 50012
Patent: Traitement du cancer de la prostate au stade métastasique par le degarelix (Treatment of metastatic stage prostate cancer with degarelix)
Estimated Expiration: ⤷ Get Started Free
Patent: 99085
Patent: Procédé de traitement du cancer de la prostate avec un antagoniste GnRH (Method Of Treating Prostate Cancer With GnRH Antagonist)
Estimated Expiration: ⤷ Get Started Free
Patent: 60565
Patent: PROCÉDÉ DE TRAITEMENT DU CANCER DE LA PROSTATE AVEC DEGARELIX (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 57197
Patent: PROCÉDÉ DE TRAITEMENT DU CANCER DE LA PROSTATE AVEC DEGARELIX (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 45011
Patent: 用地加瑞克治療轉移階段前列腺癌 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 76552
Patent: 使用 拮抗劑地加瑞克治療前列腺癌的方法 (METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX GNRH)
Estimated Expiration: ⤷ Get Started Free
Patent: 90912
Patent: 用地加瑞克治療轉移性前列腺癌 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 98243
Patent: 拮抗劑治療前列腺癌的方法 (METHOD OF TREATING PROSTATE CANCER WITH GNRH ANTAGONIST GnRH)
Estimated Expiration: ⤷ Get Started Free
Patent: 58957
Patent: 用地加瑞克治療轉移階段前列腺癌 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7295
Patent: הרכב המכיל דגארליקס לשימוש בטיפול של סרטן הערמונית בפרט שאובחן כבעל סיכון למחלת לב-דם (Composition comprising degarelix for use in the treatment of prostate cancer in a subject identified as being at risk for cardiovascular disease)
Estimated Expiration: ⤷ Get Started Free
Patent: 7400
Patent: תרכובת המכילה דג'רליקס לשימוש בטיפול בסרטן הערמונית (Composition comprising degarelix for use in the treatment of prostate cancer)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 76652
Estimated Expiration: ⤷ Get Started Free
Patent: 24866
Estimated Expiration: ⤷ Get Started Free
Patent: 89234
Estimated Expiration: ⤷ Get Started Free
Patent: 54042
Estimated Expiration: ⤷ Get Started Free
Patent: 18967
Estimated Expiration: ⤷ Get Started Free
Patent: 04003
Estimated Expiration: ⤷ Get Started Free
Patent: 18849
Estimated Expiration: ⤷ Get Started Free
Patent: 86748
Estimated Expiration: ⤷ Get Started Free
Patent: 00029
Estimated Expiration: ⤷ Get Started Free
Patent: 11511785
Estimated Expiration: ⤷ Get Started Free
Patent: 11511786
Estimated Expiration: ⤷ Get Started Free
Patent: 14141505
Patent: METHOD OF TREATING PROSTATE CANCER WITH GNRH ANTAGONIST DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 14167009
Patent: METHODS FOR TREATMENT OF METASTATIC STAGE PROSTATE CANCER
Estimated Expiration: ⤷ Get Started Free
Patent: 16193910
Patent: 転移期前立腺癌を治療する方法 (METHOD OF TREATING METASTATIC STAGE PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 16216455
Patent: GNRHアンタゴニストであるデガレリクスを用いる前立腺癌の治療方法 (METHOD OF TREATING PROSTATE CANCER USING GNRH ANTAGONIST, DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 18039814
Patent: 転移期前立腺癌を治療する方法 (METHODS FOR TREATING METASTATIC STAGE PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 19059726
Patent: 転移期前立腺癌を治療する方法 (METHODS OF TREATING METASTATIC STAGE PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 19218360
Patent: GNRHアンタゴニストであるデガレリクスを用いる前立腺癌の治療方法 (METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 20196722
Patent: 転移期前立腺癌を治療する方法 (METHOD OF TREATING METASTATIC STAGE PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 22133426
Patent: GNRHアンタゴニストであるデガレリクスを用いる前立腺癌の治療方法
Estimated Expiration: ⤷ Get Started Free
Patent: 22184898
Patent: 転移期前立腺癌を治療する方法
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0090061
Patent: طريقة معالجة سرطان البروستاتا بمضادات الهرمونات التناسلية GnRH (METHODS OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 25
Patent: علاج سرطان البروستاتا في المرحلة النقيلية بدواء ديجاريليكس (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10008816
Patent: METODO PARA TRATAR EL CANCER DE PROSTATA CON EL ANTAGONISTA DE GNRH DEGARELIX. (METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10008817
Patent: TRATAMIENTO DE CANCER DE PROSTATA EN ETAPA METASTATICA CON DEGARELIX. (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7057
Patent: METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 7088
Patent: TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 3932
Patent: Treatment of metastatic stage prostate cancer with degarelix
Estimated Expiration: ⤷ Get Started Free
Patent: 3958
Patent: Method of treating a musculoskeletal disorder or connective tissue disorder in a subject with prostate cancer with the GNRH antagonist Degarelix
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 49859
Estimated Expiration: ⤷ Get Started Free
Patent: 05204
Estimated Expiration: ⤷ Get Started Free
Patent: 50012
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 49859
Estimated Expiration: ⤷ Get Started Free
Patent: 05204
Estimated Expiration: ⤷ Get Started Free
Patent: 50012
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 04393
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ (METHOD OF TREATING PROSTATE CANCER WITH DEGARELIX THAT IS GONADOTROPIN-RELEASING HORMONE (GnRH) ANTAGONIST)
Estimated Expiration: ⤷ Get Started Free
Patent: 04394
Patent: ЛЕЧЕНИЕ МЕТАСТАТИЧЕСКОЙ СТАДИИ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ ДЕГАРЕЛИКСОМ (DEGARELIX THERAPY OF METASTATIC STAGE OF PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 10133480
Patent: ЛЕЧЕНИЕ МЕТАСТАТИЧЕСКОЙ СТАДИИ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
Patent: 10133481
Patent: СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГАРМОНА (GNRH) ДЕГАРЕЛИКСОМ
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 49859
Estimated Expiration: ⤷ Get Started Free
Patent: 05204
Estimated Expiration: ⤷ Get Started Free
Patent: 50012
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1005697
Patent: TREATMENT OF METASTATIC PROSTATE CANCER WITH DEGARELIX
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1542480
Estimated Expiration: ⤷ Get Started Free
Patent: 100123714
Patent: TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 100126362
Patent: METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 140130757
Patent: METHOD OF TREATING PROSTATE CANCER WITH THE GNRH ANTAGONIST DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 150091543
Patent: TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX
Estimated Expiration: ⤷ Get Started Free
Patent: 180118830
Patent: 데가렐릭스를 이용한 전이 단계의 전립선암의 치료 방법 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 200001623
Patent: 데가렐릭스를 이용한 전이 단계의 전립선암의 치료 방법 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 210005323
Patent: 데가렐릭스를 이용한 전이 단계의 전립선암의 치료 방법 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 220009504
Patent: 데가렐릭스를 이용한 전이 단계의 전립선암의 치료 방법 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Patent: 230088848
Patent: 데가렐릭스를 이용한 전이 단계의 전립선암의 치료 방법 (TREATMENT OF METASTATIC STAGE PROSTATE CANCER WITH DEGARELIX)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 79441
Estimated Expiration: ⤷ Get Started Free
Patent: 32709
Estimated Expiration: ⤷ Get Started Free
Patent: 40235
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 42932
Estimated Expiration: ⤷ Get Started Free
Patent: 39959
Estimated Expiration: ⤷ Get Started Free
Patent: 0938217
Patent: Method of treating metastatic stage prostate cancer
Estimated Expiration: ⤷ Get Started Free
Patent: 0938218
Patent: Methods of treating prostate cancer with GnRH antagonist
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering FIRMAGON around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 5876652 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 201300741 | СПОСОБ ЛЕЧЕНИЯ РАКА ПРЕДСТАТЕЛЬНОЙ ЖЕЛЕЗЫ АНТАГОНИСТОМ ГОНАДОТРОПИН-ВЫСВОБОЖДАЮЩЕГО ГОРМОНА (GnRH) ДЕГАРЕЛИКСОМ | ⤷ Get Started Free |
| Japan | 4249806 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for FIRMAGON
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1003774 | CA 2009 00022 | Denmark | ⤷ Get Started Free | |
| 1003774 | PA2009005,C1003774 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: DEGARELIXUM ACETAT; REGISTRATION NO/DATE: EU/1/08/504/001, 2009 02 17 EU/1/08/504/002 20090217 |
| 1003774 | SPC020/2009 | Ireland | ⤷ Get Started Free | SPC020/2009: 20091119, EXPIRES: 20230412 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for FIRMAGON (Degarelix)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
