EXONDYS 51 Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Exondys 51, and when can generic versions of Exondys 51 launch?
Exondys 51 is a drug marketed by Sarepta Theraps Inc and is included in one NDA. There are eight patents protecting this drug.
This drug has one hundred and twenty-eight patent family members in twenty-three countries.
The generic ingredient in EXONDYS 51 is eteplirsen. One supplier is listed for this compound. Additional details are available on the eteplirsen profile page.
DrugPatentWatch® Generic Entry Outlook for Exondys 51
Exondys 51 was eligible for patent challenges on September 19, 2020.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 27, 2028. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EXONDYS 51?
- What are the global sales for EXONDYS 51?
- What is Average Wholesale Price for EXONDYS 51?
Summary for EXONDYS 51
| International Patents: | 128 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 2 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EXONDYS 51 |
| What excipients (inactive ingredients) are in EXONDYS 51? | EXONDYS 51 excipients list |
| DailyMed Link: | EXONDYS 51 at DailyMed |
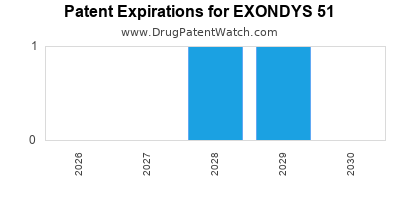
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for EXONDYS 51
Generic Entry Date for EXONDYS 51*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for EXONDYS 51
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sarepta Therapeutics | Phase 3 |
| Sarepta Therapeutics, Inc. | Phase 3 |
| Catabasis Pharmaceuticals | Phase 1/Phase 2 |
Pharmacology for EXONDYS 51
| Drug Class | Antisense Oligonucleotide |
US Patents and Regulatory Information for EXONDYS 51
EXONDYS 51 is protected by thirteen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of EXONDYS 51 is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-001 | Sep 19, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-002 | Sep 19, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-001 | Sep 19, 2016 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EXONDYS 51
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-002 | Sep 19, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-002 | Sep 19, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| Sarepta Theraps Inc | EXONDYS 51 | eteplirsen | SOLUTION;INTRAVENOUS | 206488-001 | Sep 19, 2016 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for EXONDYS 51
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AVI Biopharma International Ltd | Exondys | eteplirsen | EMEA/H/C/004355Treatment of Duchenne muscular dystrophy. | Refused | no | no | yes | 2018-12-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for EXONDYS 51
When does loss-of-exclusivity occur for EXONDYS 51?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08317566
Patent: Means and methods for counteracting muscle disorders
Estimated Expiration: ⤷ Get Started Free
Patent: 09310557
Patent: Methods and means for efficient skipping of exon 45 in Duchenne Muscular Dystrophy pre-mRNA
Estimated Expiration: ⤷ Get Started Free
Patent: 09310558
Patent: Methods and means for efficient skipping of at least one of the following exons of the human Duchenne muscular dystrophy gene: 43, 46, 50- 53.
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 04049
Patent: MOYENS ET PROCEDES POUR CONTREBALANCER DES TROUBLES MUSCULAIRES (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 41629
Patent: PROCEDES ET MOYENS D'INDUCTION DU SAUT DE L'EXON 45 DANS L'ARN PRE-MESSAGER DU GENE DE LA DYSTROPHIE MUSCULAIRE DE DUCHENNE (METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 45 IN DUCHENNE MUSCULAR DYSTROPHY PRE-MRNA)
Estimated Expiration: ⤷ Get Started Free
Patent: 41793
Patent: METHODES ET MOYENS POUR SAUTER EFFICACEMENT AU MOINS L'UN DES EXONS SUIVANTS DU GENE DE LA DYSTROPHIE MUSCULAIRE HUMAINE DE DUCHENNE : 43, 46, 50 A 53 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST ONE OF THE FOLLOWING EXONS OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE: 43, 46, 50- 53)
Estimated Expiration: ⤷ Get Started Free
Patent: 17539
Patent: METHODES ET MOYENS POUR SAUTER EFFICACEMENT AU MOINS L'UN DES EXONS SUIVANTS DU GENE DE LA DYSTROPHIE MUSCULAIRE HUMAINE DE DUCHENNE : 43, 46, 50 A 53. (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST ONE OF THE FOLLOWING EXONS OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE: 43, 46, 50-53.)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1896186
Patent: Means and methods for counteracting muscle disorders
Estimated Expiration: ⤷ Get Started Free
Patent: 2256606
Patent: Methods and means for efficient skipping of at least one of the following exons of the human duchenne muscular dystrophy gene: 43, 46, 50- 53.
Estimated Expiration: ⤷ Get Started Free
Patent: 2264903
Estimated Expiration: ⤷ Get Started Free
Patent: 5641700
Patent: 对抗肌肉病症的方式和方法 (Means and methods for counteracting muscle disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 5647921
Patent: 有效跳跃人杜兴肌营养不良基因外显子43、46、50-53中至少个的方法和手段 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST ONE EXONS 43, 46, 50-53 IN HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160025
Estimated Expiration: ⤷ Get Started Free
Patent: 0160078
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16305
Estimated Expiration: ⤷ Get Started Free
Patent: 17286
Estimated Expiration: ⤷ Get Started Free
Patent: 17454
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 03173
Estimated Expiration: ⤷ Get Started Free
Patent: 44637
Estimated Expiration: ⤷ Get Started Free
Patent: 07484
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 03173
Patent: MOYENS ET PROCÉDÉ DE COMPENSATION DES TROUBLES MUSCULAIRES (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 44637
Patent: PROCÉDÉS ET MOYENS D INDUCTION DU SAUT DE L EXON 45 DANS L ARN PRÉ-MESSAGER DU GÈNE DE LA DYSTROPHIE MUSCULAIRE DE DUCHENNE (METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 45 IN DUCHENNE MUSCULAR DYSTROPHY PRE-MRNA)
Estimated Expiration: ⤷ Get Started Free
Patent: 49287
Patent: MÉTHODES ET MOYENS POUR SAUTER EFFICACEMENT AU MOINS L`UN DES EXONS SUIVANTS DU GÈNE DE LA DYSTROPHIE MUSCULAIRE HUMAINE DE DUCHENNE : 43, 46, 50 À 53 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST ONE OF THE FOLLOWING EXONS OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE: 43, 46, 50- 53.)
Estimated Expiration: ⤷ Get Started Free
Patent: 07484
Patent: Procédé et moyens d’induction du saut de l’exon 45 dans l’ARN pré-messager du gène de la dystrophie musculaire de Duchenne (Methods and means for efficient skipping of exon 45 in Duchenne Muscular Dystrophy pre-mRNA)
Estimated Expiration: ⤷ Get Started Free
Patent: 14827
Patent: Moyens et procédé de compensation des troubles musculaires (Means and methods for counteracting muscle disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 38737
Patent: MOYENS ET PROCÉDÉ DE COMPENSATION DES TROUBLES MUSCULAIRES (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 00948
Patent: PROCÉDÉS ET MOYENS POUR SAUTER EFFICACEMENT AU MOINS L`UN DES EXONS SUIVANTS DU GÈNE DE LA DYSTROPHIE MUSCULAIRE HUMAINE DE DUCHENNE : 43, 46, 50 À 53 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST ONE OF THE FOLLOWING EXONS OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE: 43, 46, 50- 53)
Estimated Expiration: ⤷ Get Started Free
Patent: 83399
Patent: PROCÉDÉS ET MOYENS POUR SAUTER EFFICACEMENT AU MOINS L'EXON 52 DU GÈNE DE LA DYSTROPHIE MUSCULAIRE HUMAINE DE DUCHENNE (METHODS AND MEANS FOR EFFICIENT SKIPPING OF AT LEAST EXON 52 OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 60169
Patent: 高效跳過杜氏肌營養不良症 前體 中的外顯子 的均數和方法 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 45 IN DUCHENNE MUSCULAR DYSTROPHY PRE-MRNA (DMD) MRNA 45)
Estimated Expiration: ⤷ Get Started Free
Patent: 85098
Patent: 高效跳過裘馨氏肌肉營養不良症前體 外顯子 的方法和工具 (METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 45 IN DUCHENNE MUSCULAR DYSTROPHY PRE-MRNA MRNA 45)
Estimated Expiration: ⤷ Get Started Free
Patent: 45670
Patent: 對抗肌肉病症的裝置和方法 (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27124
Estimated Expiration: ⤷ Get Started Free
Patent: 28662
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5322
Patent: שילוב של אוליגונוקלאוטיד משלים ל, pre-mrna- של אקסון 51 דיסטרופין וסטרואיד, לשימוש כתרופה ותכשירים רפואיים המכילים אותו (Combination of an oligonucleotide complementary to pre-mrna of dystrophin exon 51 and a steroid, for use as a medicament and pharmaceutical preparations thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 2508
Patent: אמצעים להשראת קפיצה באקסון 45 בפרה mrna של דיסטרופין על ידי אנטיסנס אוליקונקלאוטיד ושימושיהם (Means for efficient skipping of exon 45 in dystrophin pre-mrna using an antisense oligonucleotide and uses thereof)
Estimated Expiration: ⤷ Get Started Free
Patent: 2509
Patent: אוליגונוקליאוטידים אנטיסנס ושימושם להשראת פסיחה באקסון 52 של הגן ההומאני dystrophy muscular duchenne (Antisense oligonucleotides and the use of same for efficient skipping of exon 52 of the human duchenne muscular dystrophy gene)
Estimated Expiration: ⤷ Get Started Free
Patent: 1928
Patent: Combination pharmaceutical preparation comprising an oligonucleotide and an ion channel inhibitor for alleviating symptoms of duchenne muscular dystrophy (dmd) or becker muscular dystrophy (bmd)
Estimated Expiration: ⤷ Get Started Free
Patent: 1127
Patent: תכשירים ושיטות לטיפול במחלות שרירים (Means and methods for counteracting muscle disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 5424
Patent: תכשירים ושיטות לטיפול במחלות שרירים (Means and methods for counteracting muscle disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 4321
Patent: תכשירים ושיטות לטיפול במחלות שרירים (Means and methods for counteracting muscle disorders)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 00064
Estimated Expiration: ⤷ Get Started Free
Patent: 86109
Estimated Expiration: ⤷ Get Started Free
Patent: 79374
Estimated Expiration: ⤷ Get Started Free
Patent: 05260
Estimated Expiration: ⤷ Get Started Free
Patent: 79629
Estimated Expiration: ⤷ Get Started Free
Patent: 85620
Estimated Expiration: ⤷ Get Started Free
Patent: 07622
Estimated Expiration: ⤷ Get Started Free
Patent: 11502118
Estimated Expiration: ⤷ Get Started Free
Patent: 12506697
Estimated Expiration: ⤷ Get Started Free
Patent: 12506698
Estimated Expiration: ⤷ Get Started Free
Patent: 14111638
Patent: MEANS AND METHOD FOR COUNTERACTING MUSCLE DISORDERS
Estimated Expiration: ⤷ Get Started Free
Patent: 16033140
Patent: 筋障害を相殺するための手段と方法 (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 17141296
Patent: 筋障害を相殺するための手段と方法 (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19142942
Patent: 筋障害を相殺するための手段と方法 (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 21113229
Patent: 筋障害を相殺するための手段と方法 (MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4793
Patent: MEANS AND METHODS FOR COUNTERACTING MUSCLE DISORDERS
Estimated Expiration: ⤷ Get Started Free
Patent: 2446
Patent: METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 45 IN DUCHENNE MUSCULAR DYSTROPHY PRE-MRNA
Estimated Expiration: ⤷ Get Started Free
Patent: 2498
Patent: METHODS AND MEANS FOR EFFICIENT SKIPPING OF EXON 52 OF THE HUMAN DUCHENNE MUSCULAR DYSTROPHY GENE
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 03173
Estimated Expiration: ⤷ Get Started Free
Patent: 07484
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 03173
Estimated Expiration: ⤷ Get Started Free
Patent: 44637
Estimated Expiration: ⤷ Get Started Free
Patent: 07484
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 07484
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 32634
Estimated Expiration: ⤷ Get Started Free
Patent: 62658
Estimated Expiration: ⤷ Get Started Free
Patent: 64563
Estimated Expiration: ⤷ Get Started Free
Patent: 39852
Estimated Expiration: ⤷ Get Started Free
Patent: 92886
Estimated Expiration: ⤷ Get Started Free
Patent: 14775
Estimated Expiration: ⤷ Get Started Free
Patent: 36464
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EXONDYS 51 around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2010505741 | ⤷ Get Started Free | |
| European Patent Office | 2607484 | ⤷ Get Started Free | |
| European Patent Office | 3238737 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for EXONDYS 51
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
