EVAMIST Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Evamist, and what generic alternatives are available?
Evamist is a drug marketed by Padagis Us and is included in one NDA.
The generic ingredient in EVAMIST is estradiol. There are seventy-five drug master file entries for this compound. Forty-six suppliers are listed for this compound. Additional details are available on the estradiol profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Evamist
A generic version of EVAMIST was approved as estradiol by BARR LABS INC on October 22nd, 1997.
Summary for EVAMIST
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 1 |
| Patent Applications: | 4,323 |
| Formulation / Manufacturing: | see details |
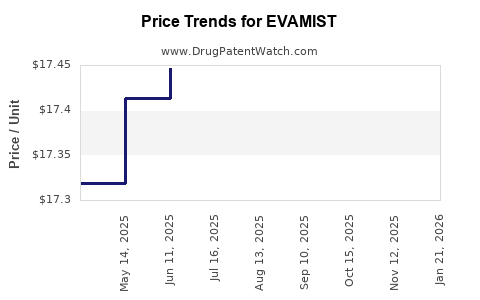
| Drug Prices: | Drug price information for EVAMIST |
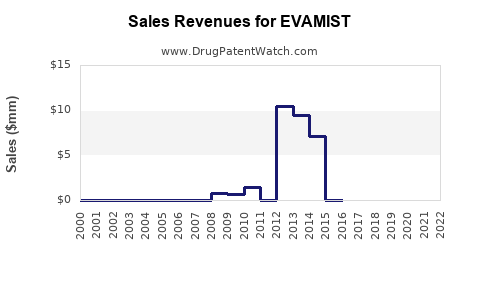
| Drug Sales Revenues: | Drug sales revenues for EVAMIST |
| What excipients (inactive ingredients) are in EVAMIST? | EVAMIST excipients list |
| DailyMed Link: | EVAMIST at DailyMed |
Recent Clinical Trials for EVAMIST
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Lumara Health, Inc. | Phase 3 |
Pharmacology for EVAMIST
| Drug Class | Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for EVAMIST
US Patents and Regulatory Information for EVAMIST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Padagis Us | EVAMIST | estradiol | SPRAY;TRANSDERMAL | 022014-001 | Jul 27, 2007 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EVAMIST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Padagis Us | EVAMIST | estradiol | SPRAY;TRANSDERMAL | 022014-001 | Jul 27, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Padagis Us | EVAMIST | estradiol | SPRAY;TRANSDERMAL | 022014-001 | Jul 27, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Padagis Us | EVAMIST | estradiol | SPRAY;TRANSDERMAL | 022014-001 | Jul 27, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Padagis Us | EVAMIST | estradiol | SPRAY;TRANSDERMAL | 022014-001 | Jul 27, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for EVAMIST
See the table below for patents covering EVAMIST around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1087355 | ⤷ Try a Trial | |
| Canada | 2436882 | ⤷ Try a Trial | |
| Germany | 122012000019 | ⤷ Try a Trial | |
| Canada | 2244089 | PROMOTEURS DE PENETRATION DERMIQUE ET SYSTEME D'ADMINISTRATION DE MEDICAMENTS COMPRENANT CES PROMOTEURS (DERMAL PENETRATION ENHANCERS AND DRUG DELIVERY SYSTEMS INVOLVING SAME) | ⤷ Try a Trial |
| Hong Kong | 1101553 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EVAMIST
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1769785 | C300521 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL; REG NO/DATE: EU/2/11/127/001 20111006 |
| 2782584 | 122021000080 | Germany | ⤷ Try a Trial | PRODUCT NAME: ZUSAMMENSETZUNG, DIE SOWOHL ESTRADIOL (17SS-ESTRADIOL), GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, HYDRATS ODER SOLVATS DAVON (EINSCHLIESSLICH IN FORM EINES HEMIHYDRATS), ALS AUCH PROGESTERON ENTHAELT; NAT. REGISTRATION NO/DATE: 2205034.00.00 20210924; FIRST REGISTRATION: BELGIEN BE582231 20210406 |
| 0398460 | 12/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON IN KOMBINATION MIT ESTRADIOL; NAT. REGISTRATION NO/DATE: 1-25178, 1-25179 20031127; FIRST REGISTRATION: NL RVG 27505 20021211 |
| 0770388 | 2009/012 | Ireland | ⤷ Try a Trial | PRODUCT NAME: QLAIRA-ESTRADIOL VALERATE/DIENOGEST; NAT REGISTRATION NO/DATE: PA1410/58/1 20090109; FIRST REGISTRATION NO/DATE: BE327792 20081103 |
| 0901368 | C300523 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL; REGISTRATION NO/DATE: EU/2/11/127/001 20111006 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


