DALVANCE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Dalvance, and what generic alternatives are available?
Dalvance is a drug marketed by Abbvie and is included in one NDA. There is one patent protecting this drug.
This drug has fifty-eight patent family members in twenty countries.
The generic ingredient in DALVANCE is dalbavancin hydrochloride. One supplier is listed for this compound. Additional details are available on the dalbavancin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Dalvance
Dalvance was eligible for patent challenges on May 23, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 22, 2024. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for DALVANCE
| International Patents: | 58 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 3 |
| Clinical Trials: | 6 |
| Patent Applications: | 5 |
| Drug Prices: | Drug price information for DALVANCE |
| What excipients (inactive ingredients) are in DALVANCE? | DALVANCE excipients list |
| DailyMed Link: | DALVANCE at DailyMed |
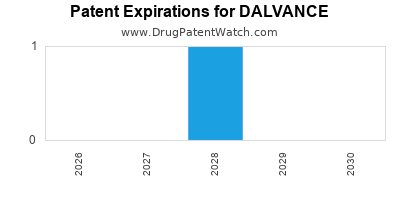

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DALVANCE
Generic Entry Date for DALVANCE*:
Constraining patent/regulatory exclusivity:
NEW PATIENT POPULATION NDA:
Dosage:
POWDER;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DALVANCE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wake Forest University Health Sciences | Phase 4 |
| University of Colorado, Denver | Phase 2/Phase 3 |
| Washington University School of Medicine | Phase 4 |
Pharmacology for DALVANCE
| Drug Class | Lipoglycopeptide Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for DALVANCE
US Patents and Regulatory Information for DALVANCE
DALVANCE is protected by one US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of DALVANCE is ⤷ Try a Trial.
This potential generic entry date is based on NEW PATIENT POPULATION.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting DALVANCE
Methods of administering dalbavancin for treatment of bacterial infections
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTIONS (ABSSSI) IN ADULT AND PEDIATRIC PATIENTS USING A TWO-DOSE REGIMEN OF DALBAVANCIN
FDA Regulatory Exclusivity protecting DALVANCE
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Try a Trial
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DALVANCE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | DALVANCE | dalbavancin hydrochloride | POWDER;INTRAVENOUS | 021883-001 | May 23, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DALVANCE
See the table below for patents covering DALVANCE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1114014 | PHARMACEUTICAL COMPOSITION COMPRISING DALBAVANCIN | ⤷ Try a Trial |
| Russian Federation | 2006136736 | КОМПОЗИЦИИ ДАЛБАВАНЦИНА ДЛЯ ЛЕЧЕНИЯ БАКТЕРИАЛЬНЫХИНФЕКЦИЙ | ⤷ Try a Trial |
| South Africa | 200607888 | Dalbavancin compositions for treatment of bacterial infections | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


