COPIKTRA Drug Patent Profile
✉ Email this page to a colleague
When do Copiktra patents expire, and what generic alternatives are available?
Copiktra is a drug marketed by Secura and is included in one NDA. There are six patents protecting this drug.
This drug has two hundred and twenty-seven patent family members in thirty-nine countries.
The generic ingredient in COPIKTRA is duvelisib. One supplier is listed for this compound. Additional details are available on the duvelisib profile page.
DrugPatentWatch® Generic Entry Outlook for Copiktra
Copiktra was eligible for patent challenges on September 24, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 17, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for COPIKTRA?
- What are the global sales for COPIKTRA?
- What is Average Wholesale Price for COPIKTRA?
Summary for COPIKTRA
| International Patents: | 227 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 69 |
| Clinical Trials: | 12 |
| Drug Prices: | Drug price information for COPIKTRA |
| What excipients (inactive ingredients) are in COPIKTRA? | COPIKTRA excipients list |
| DailyMed Link: | COPIKTRA at DailyMed |
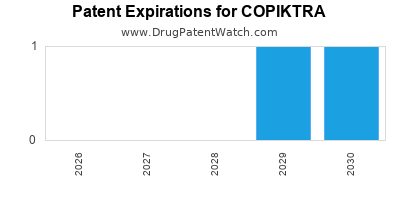

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for COPIKTRA
Generic Entry Date for COPIKTRA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for COPIKTRA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| CSPC ZhongQi Pharmaceutical Technology Co., Ltd. | Phase 1/Phase 2 |
| Secura Bio, Inc. | Early Phase 1 |
| National Cancer Institute (NCI) | Early Phase 1 |
US Patents and Regulatory Information for COPIKTRA
COPIKTRA is protected by eight US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of COPIKTRA is ⤷ Get Started Free.
This potential generic entry date is based on patent RE46621.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secura | COPIKTRA | duvelisib | CAPSULE;ORAL | 211155-001 | Sep 24, 2018 | RX | Yes | No | 9,840,505 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Secura | COPIKTRA | duvelisib | CAPSULE;ORAL | 211155-002 | Sep 24, 2018 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Secura | COPIKTRA | duvelisib | CAPSULE;ORAL | 211155-001 | Sep 24, 2018 | RX | Yes | No | RE46621 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Secura | COPIKTRA | duvelisib | CAPSULE;ORAL | 211155-001 | Sep 24, 2018 | RX | Yes | No | 12,213,983 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Secura | COPIKTRA | duvelisib | CAPSULE;ORAL | 211155-001 | Sep 24, 2018 | RX | Yes | No | 8,193,182 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for COPIKTRA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Secura Bio Limited | Copiktra | duvelisib | EMEA/H/C/005381Copiktra monotherapy is indicated for the treatment of adult patients with: Relapsed or refractory chronic lymphocytic leukaemia (CLL) after at least two prior therapies. Follicular lymphoma (FL) that is refractory to at least two prior systemic therapies. | Authorised | no | no | no | 2021-05-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for COPIKTRA
When does loss-of-exclusivity occur for COPIKTRA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4824
Patent: PROCESOS PARA PREPARAR ISOQUINOLINONAS Y FORMAS SOLIDAS DE ISOQUINOLINONAS
Estimated Expiration: ⤷ Get Started Free
Patent: 7467
Patent: PROCESOS PARA PREPARAR ISOQUINOLINONAS Y FORMAS SÓLIDAS DE ISOQUINOLINONAS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12205669
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Patent: 15258280
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013017670
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 24197
Patent: PROCEDES DE PREPARATION D'ISOQUINOLINONES ET DE FORMES SOLIDES D'ISOQUINOLINONES (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 13002007
Patent: Compuesto polimorfo (s)-3-(1-(9h-purin-6-ilamino)etil)-8-cloro-2-fenilisoquinolin-1-(2h)-ona de formas b-j, amorfa, sal, solvato o hidrato del mismo; mezclas de estos compuestos; metodo para preparar el polimorfo de forma c; composicion farmaceutica; metodo de tratamiento; uso para el tratamiento de un trastorno mediado por pi3k.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3648499
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 63309
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 63309
Patent: PROCÉDÉS DE PRÉPARATION D'ISOQUINOLINONES ET DE FORMES SOLIDES D'ISOQUINOLINONES (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Patent: 38722
Patent: FORMES SOLIDES D'ISOQUINOLINONES (SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Patent: 81574
Patent: COMPOSITION POUR ADMINISTRATION ORALE À UTILISER DANS LE TRAITEMENT DU CANCER, D'UNE MALADIE INFLAMMATOIRE OU D'UNE MALADIE AUTO-IMMUNE (A COMPOSITION FOR ORAL ADMINISTRATION FOR USE IN THE TREATMENT OF CANCER, AN INFLAMMATORY DISEASE OR AN AUTO-IMMUNE DISEASE)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 45110
Patent: 異喹啉酮的固體形式 (SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7387
Patent: תהליכים להכנת איזוקווינולינונים וצורת מוצקות של איזוקווינולינונים (Processes for preparing isoquinolinones and solid forms of isoquinolinones)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 54672
Estimated Expiration: ⤷ Get Started Free
Patent: 14501790
Estimated Expiration: ⤷ Get Started Free
Patent: 17061547
Patent: イソキノリノンの調製方法及びイソキノリノンの固体形態 (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 7708
Patent: PROCESO PARA PREPARAR ISOQUINOLINONAS Y FORMAS SOLIDAS DE ISOQUINOLINONAS. (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13008065
Patent: PROCESO PARA PREPARAR ISOQUINOLINONAS Y FORMAS SOLIDAS DE ISOQUINOLINONAS. (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2909
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 141303
Patent: PROCEDIMIENTO PARA PREPARAR ISOQUINOLINONAS Y FORMAS SOLIDAS DE ISOQUINOLINONAS
Estimated Expiration: ⤷ Get Started Free
Patent: 180318
Patent: PROCEDIMIENTO PARA PREPARAR ISOQUINOLINONAS Y FORMAS SOLIDAS DE ISOQUINOLINONAS
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 018500960
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 26883
Patent: СПОСОБЫ ПОЛУЧЕНИЯ ИЗОХИНОЛИНОНОВ И ТВЕРДЫЕ ФОРМЫ ИЗОХИНОЛИНОНОВ (METHODS OF PRODUCTION ISOQUINOLINONES AND SOLID FORMS ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Patent: 13137424
Patent: СПОСОБЫ ПОЛУЧЕНИЯ ИЗОХИНОЛИНОНОВ И ТВЕРДЫЕ ФОРМЫ ИЗОХИНОЛИНОНОВ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1897
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
Patent: 201600179R
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1305150
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1875720
Estimated Expiration: ⤷ Get Started Free
Patent: 140020249
Patent: PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES
Estimated Expiration: ⤷ Get Started Free
Patent: 180080358
Patent: 이소퀴놀린온 및 이의 고체 형태의 제조 방법 (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 37113
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 46305
Estimated Expiration: ⤷ Get Started Free
Patent: 59956
Estimated Expiration: ⤷ Get Started Free
Patent: 74262
Estimated Expiration: ⤷ Get Started Free
Patent: 1247670
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Patent: 1700475
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Patent: 1906841
Patent: Processes for preparing isoquinolinones and solid forms of isoquinolinones
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 5767
Patent: СПОСОБИ ОТРИМАННЯ ІЗОХІНОЛІНОНІВ І ТВЕРДІ ФОРМИ ІЗОХІНОЛІНОНІВ (PROCESSES FOR PREPARING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering COPIKTRA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2914296 | ⤷ Get Started Free | |
| Taiwan | I659956 | ⤷ Get Started Free | |
| Mexico | 358640 | ⤷ Get Started Free | |
| Japan | 7088889 | ⤷ Get Started Free | |
| South Africa | 201005390 | CERTAIN CHEMICAL ENTITIES,COMPOSITIONS AND METHODS | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for COPIKTRA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2456444 | 811 | Finland | ⤷ Get Started Free | |
| 2456444 | SPC/GB21/069 | United Kingdom | ⤷ Get Started Free | SPC/GB21/069 WAS ADDED TO THE JOURNAL 7041 INCORRECTLY IDENTIFYING THE INCORRECT MAXIMUM EXPIRY DATE ON WHICH THE SPC IS BASED. PLEASE SEE THE UPDATED JOURNAL ENTRY.; APPLICANT: INTELLIKINE, LLC; 10931 NORTH TORREY PINES ROAD, LA JOLLA CA 92037, UNITED STATES OF AMERICA; PRODUCT: DUVELISIB; PRODUCT TYPE: MEDICINAL; AUTHORISED:; EU/1/21/1542/001 21 MAY 2021 (NI); EU/1/21/1542/002 21 MAY 2021 (NI) ; FURTHER MA'S ON IPSUM; ; AUTHORISED EXTENSION:; PATENT NO: EP2456444; TITLE: ADENINE DERIVATIVE AS PI3K INHIBITOR; SPC NO: SPC/GB21/069; DATE GRANTED: 05 APRIL 2024; MAXIMUM PERIOD EXPIRES ON: 14 JULY 2035; CORRECTED DATA |
| 2456444 | C20210033 00435 | Estonia | ⤷ Get Started Free | PRODUCT NAME: DUVELISIIB;REG NO/DATE: EU/1/21/1542 21.05.2021 |
| 2456444 | 301140 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: DUVELISIB; REGISTRATION NO/DATE: EU/1/21/1542 20210521 |
| 2914296 | 47/2021 | Austria | ⤷ Get Started Free | PRODUCT NAME: DUVELISIB; REGISTRATION NO/DATE: EU/1/21/1542 (MITTEILUNG) 20210521 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for COPIKTRA (Duvelisib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
