BALCOLTRA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Balcoltra, and what generic alternatives are available?
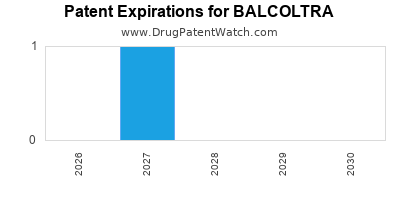
Balcoltra is a drug marketed by Avion Pharms and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has three patent family members in three countries.
The generic ingredient in BALCOLTRA is ethinyl estradiol; levonorgestrel. There are twenty-six drug master file entries for this compound. Twenty-six suppliers are listed for this compound. Additional details are available on the ethinyl estradiol; levonorgestrel profile page.
DrugPatentWatch® Generic Entry Outlook for Balcoltra
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
There are two tentative approvals for the generic drug (ethinyl estradiol; levonorgestrel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for BALCOLTRA
| International Patents: | 3 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Formulation / Manufacturing: | see details |
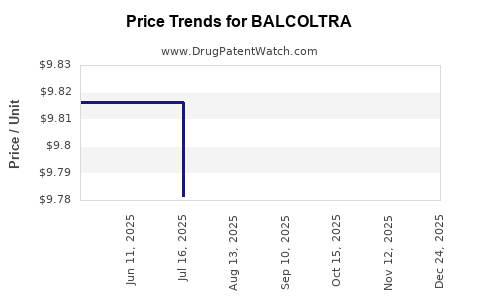
| Drug Prices: | Drug price information for BALCOLTRA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BALCOLTRA |
| What excipients (inactive ingredients) are in BALCOLTRA? | BALCOLTRA excipients list |
| DailyMed Link: | BALCOLTRA at DailyMed |
Anatomical Therapeutic Chemical (ATC) Classes for BALCOLTRA
Paragraph IV (Patent) Challenges for BALCOLTRA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BALCOLTRA | Tablets | ethinyl estradiol; levonorgestrel | 0.1 mg/0.02 mg | 208612 | 1 | 2020-07-14 |
US Patents and Regulatory Information for BALCOLTRA
BALCOLTRA is protected by one US patents.
Patents protecting BALCOLTRA
Hypoallergenic metal amino acid chelates and metal amino acid chelate-containing compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADMINISTRATION OF FERROUS BISGLYCINATE TABLETS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avion Pharms | BALCOLTRA | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 208612-001 | Jan 9, 2018 | AB3 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BALCOLTRA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Avion Pharms | BALCOLTRA | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 208612-001 | Jan 9, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BALCOLTRA
See the table below for patents covering BALCOLTRA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| United Kingdom | 2394474 | Enhancing solubility of iron amino acid chelates and iron proteinates | ⤷ Try a Trial |
| Canada | 2457584 | AMELIORATION DE LA SOLUBILITE DES CHELATES D'ACIDES AMINES DE FER ET DES PROTEINATES DE FER (ENHANCING SOLUBILITY OF IRON AMINO ACID CHELATES AND IRON PROTEINATES) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005105112 | ⤷ Try a Trial | |
| Australia | 2002331603 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BALCOLTRA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1214076 | C01214076/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE + ETHINYLESTRADIOL; REGISTRATION NUMBER/DATE: SWISSMEDIC 57946 13.06.2008 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1214076 | 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON; REGISTRATION NO/DATE: 1-27586 20080612 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


