AVYCAZ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Avycaz, and when can generic versions of Avycaz launch?
Avycaz is a drug marketed by Abbvie and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and ninety-five patent family members in fifty-five countries.
The generic ingredient in AVYCAZ is avibactam sodium; ceftazidime. One supplier is listed for this compound. Additional details are available on the avibactam sodium; ceftazidime profile page.
DrugPatentWatch® Generic Entry Outlook for Avycaz
Avycaz was eligible for patent challenges on February 25, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 15, 2032. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AVYCAZ?
- What are the global sales for AVYCAZ?
- What is Average Wholesale Price for AVYCAZ?
Summary for AVYCAZ
| International Patents: | 195 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 3 |
| Patent Applications: | 96 |
| Drug Prices: | Drug price information for AVYCAZ |
| What excipients (inactive ingredients) are in AVYCAZ? | AVYCAZ excipients list |
| DailyMed Link: | AVYCAZ at DailyMed |
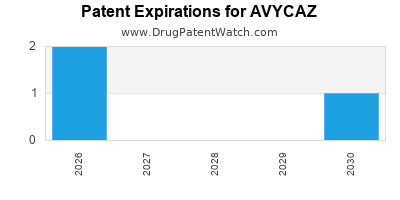

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AVYCAZ
Generic Entry Date for AVYCAZ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;IV (INFUSION) |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for AVYCAZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute of Allergy and Infectious Diseases (NIAID) | Phase 1 |
| Michigan State University | Phase 1 |
| University of Southern California | Phase 4 |
Pharmacology for AVYCAZ
| Drug Class | Cephalosporin Antibacterial beta Lactamase Inhibitor |
| Mechanism of Action | beta Lactamase Inhibitors |
Paragraph IV (Patent) Challenges for AVYCAZ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AVYCAZ | For Injection | avibactam sodium; ceftazidime | 0.5 g/2 g per vial | 206494 | 2 | 2024-02-26 |
US Patents and Regulatory Information for AVYCAZ
AVYCAZ is protected by ten US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AVYCAZ is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,969,566.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for AVYCAZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | AVYCAZ | avibactam sodium; ceftazidime | POWDER;INTRAVENOUS | 206494-001 | Feb 25, 2015 | 8,178,554 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AVYCAZ
When does loss-of-exclusivity occur for AVYCAZ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6972
Patent: PROCESOS PARA PREPARAR COMPUESTOS HETEROCICLICOS, QUE INCLUYEN TRANS-7-OXO-6-(SULFOOXI)-1,6-DIAZABICICLO[3,2,1]OCTANO-2-CARBOXAMIDA Y SALES DE LA MISMA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12270051
Patent: Process for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013032415
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 80403
Patent: PROCEDES DE PREPARATION DE COMPOSES HETEROCYCLIQUES, Y COMPRIS LE TRANS-7-OXO-6-(SULFOXY)-1,6-DIAZABICYCLO¬3,2,1|OCTANE-2-CARBOXAMIDE ET SES SELS (PROCESSES FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3649051
Patent: Process for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1, 6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 5294690
Patent: PROCESSES FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 21005
Patent: PROCÉDÉ DE PRÉPARATION DE COMPOSÉS HÉTÉROCYCLIQUES, NOTAMMENT LE TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE ET SES SELS (PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 96615
Patent: 製備包括反式- -氧- 磺酰氧基 -二氮二環 辛烷- -氨甲酰及其鹽的雜環化合物的方法 (PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6- (SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF -7--6-()-16-[321]-2-)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9815
Patent: תהליך להכנת הטרוציקליות הכוללות טרנס- 7 - אוקסו - 6 - (סולפואוקסי) - 1, 6 - דיאזאביציקלו [1,2,3] אוקטאן - 2 - קרבוקסאמיד ומלחים שלהן (Process for preparing heterocyclic compounds including trans-7-oxo- 6 -(sulphooxy)-1, 6 - diazabicyclo [3,2,1] octane- 2-carboxamide and salts thereof)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 23800
Estimated Expiration: ⤷ Get Started Free
Patent: 42462
Estimated Expiration: ⤷ Get Started Free
Patent: 14517027
Estimated Expiration: ⤷ Get Started Free
Patent: 17036307
Patent: trans−7−オキソ−6−(スルホオキシ)−1,6−ジアザビシクロ[3,2,1]オクタン−2−カルボキサミドとその塩が含まれる複素環式化合物を製造するための方法 (PROCESSES FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULFOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 5730
Patent: PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1020
Patent: PROCESOS PARA PREPARAR COMPUESTOS HETEROCICLICOS, INCLUIDA TRANS-7-OXO-6-(SULFOOXI)-1,6-DIAZABICICLO [3,2,1]OCTANO-2-CARBOXAM IDA Y SALES DE LA MISMA. (PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBO XAMIDE AND SALTS THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13014114
Patent: PROCESOS PARA PREPARAR COMPUESTOS HETEROCICLICOS, INCLUIDA TRANS-7-OXO-6-(SULFOOXI)-1,6-DIABAZABICICLO [3,2,1]OCTANO-2-CARBOX AMIDA Y SALES DE LA MISMA. (PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBO XAMIDE AND SALTS THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 10091
Estimated Expiration: ⤷ Get Started Free
Patent: 69076
Patent: СПОСОБ ПОЛУЧЕНИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ, ВКЛЮЧАЯ ТРАНС-7-ОКСО-6-(СУЛЬФОКСИ)-1,6-ДИАЗАБИЦИКЛО[3.2.1]ОКТАН-2-КАРБОКСАМИД И ЕГО СОЛИ (METHOD OF PRODUCING HETEROCYCLIC COMPOUNDS, INCLUDING TRANS-7-OXO-6-(SULPHOXY)-1,6-DIAZABICYCLO[3.2.1]OCTANE-2-CARBOXAMIDE AND ITS SALT)
Estimated Expiration: ⤷ Get Started Free
Patent: 14101244
Patent: СПОСОБ ПОЛУЧЕНИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ, ВКЛЮЧАЯ ТРАНС-7-ОКСО-6-(СУЛЬФООКСИ)-1, 6-ДИАЗАБИЦИКЛО[3,2,1]ОКТАН-2-КАРБОКСАМИД И ЕГО СОЛИ
Estimated Expiration: ⤷ Get Started Free
Patent: 17102358
Patent: СПОСОБ ПОЛУЧЕНИЯ ГЕТЕРОЦИКЛИЧЕСКИХ СОЕДИНЕНИЙ, ВКЛЮЧАЯ ТРАНС-7-ОКСО-6-(СУЛЬФООКСИ)-1,6-ДИАЗАБИцикло[3,2,1]ОКТАН-2-КАРБОКСАМИД И ЕГО СОЛИ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 5289
Patent: PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2143660
Estimated Expiration: ⤷ Get Started Free
Patent: 140040748
Patent: PROCESS FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULPHOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 60404
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 65706
Estimated Expiration: ⤷ Get Started Free
Patent: 1317238
Patent: Processes for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1-,6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AVYCAZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | I565706 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 004920 | АЗАБИЦИКЛИЧЕСКИЕ СОЕДИНЕНИЯ, СПОСОБ ИХ ПОЛУЧЕНИЯ И ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ ЛЕКАРСТВЕННЫХ СРЕДСТВ, В ЧАСТНОСТИ В КАЧЕСТВЕ АНТИБАКТЕРИАЛЬНЫХ СРЕДСТВ (AZABICYCLIC COMPOUNDS, PREPARATION THEREOF AND USE AS MEDICINES, IN PARTICULAR AS ANTIBACTERIAL AGENTS) | ⤷ Get Started Free |
| Japan | 2017036307 | trans−7−オキソ−6−(スルホオキシ)−1,6−ジアザビシクロ[3,2,1]オクタン−2−カルボキサミドとその塩が含まれる複素環式化合物を製造するための方法 (PROCESSES FOR PREPARING HETEROCYCLIC COMPOUNDS INCLUDING TRANS-7-OXO-6-(SULFOOXY)-1,6-DIAZABICYCLO[3,2,1]OCTANE-2-CARBOXAMIDE AND SALTS THEREOF) | ⤷ Get Started Free |
| South Korea | 101817811 | ⤷ Get Started Free | |
| Algeria | 3397 | COMPOSES AZABICYCLIQUES, LEUR PREPARATION ET LEUR UTILISATION COMME MEDICAMENTS, NOTAMMENT COMME ANTI-BACTERIENS | ⤷ Get Started Free |
| Japan | 2017149754 | ⤷ Get Started Free | |
| Morocco | 26938 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AVYCAZ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1480644 | SPC/GB17/004 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: COMBINATION OF CEFTAZIDIME, OR A SALT THEREOF, AND AVIBACTAM, OR A SALT THEREOF; REGISTERED: UK EU/1/16/1109/001 20160628 |
| 1480644 | 1690059-9 | Sweden | ⤷ Get Started Free | PRODUCT NAME: PHARMACEUTICAL MIXTURE OR ASSOCIATION THAT INCLUDES AS ACTIVE INGREDIENTS: (1) CEFTAZIDIME OR A SALT THEREOF, AND (2) AVIBACTAM OR A SALT THEREOF; REG. NO/DATE: EU/1/16/1109 20160628 |
| 1480644 | PA2016037,C1480644 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: FARMACINIS MISINYS ARBA DERINYS, APIMANTIS KAIP VEIKLIUOSIUS INGREDIENTUS (1) CEFTAZIDIMA ARBA JO DRUSKA IR (2) AVIBAKTAMA ARBA JO DRUSKA; REGISTRATION NO/DATE: EU/1/16/1109/001 20160624 |
| 1480644 | C20160041 00213 | Estonia | ⤷ Get Started Free | PRODUCT NAME: TSEFTASIDIIM/AVIBAKTAAM;REG NO/DATE: EU/1/16/1109 28.06.2016 |
| 1307457 | C 2016 055 | Romania | ⤷ Get Started Free | PRODUCT NAME: AVIBACTAM SAU O SARE A ACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/16/1109; DATE OF NATIONAL AUTHORISATION: 20160624; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/16/1109; DATE OF FIRST AUTHORISATION IN EEA: 20160624 |
| 1480644 | 300847 | Netherlands | ⤷ Get Started Free | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT |
| 1480644 | 339 5029-2016 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: CEFTAZIDIM VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM A AVIBAKTAM VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/16/1109 20160628 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: AVYCAZ
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
