AVYCAZ Drug Patent Profile
✉ Email this page to a colleague
When do Avycaz patents expire, and what generic alternatives are available?
Avycaz is a drug marketed by Allergan and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and ninety-five patent family members in fifty-five countries.
The generic ingredient in AVYCAZ is avibactam sodium; ceftazidime. One supplier is listed for this compound. Additional details are available on the avibactam sodium; ceftazidime profile page.
DrugPatentWatch® Generic Entry Outlook for Avycaz
Avycaz was eligible for patent challenges on February 25, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 15, 2032. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for AVYCAZ
| International Patents: | 195 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 3 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for AVYCAZ |
| What excipients (inactive ingredients) are in AVYCAZ? | AVYCAZ excipients list |
| DailyMed Link: | AVYCAZ at DailyMed |
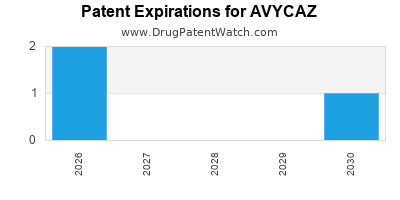

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AVYCAZ
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for AVYCAZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute of Allergy and Infectious Diseases (NIAID) | Phase 1 |
| Michigan State University | Phase 1 |
| University of Southern California | Phase 4 |
Pharmacology for AVYCAZ
| Drug Class | Cephalosporin Antibacterial beta Lactamase Inhibitor |
| Mechanism of Action | beta Lactamase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for AVYCAZ
Paragraph IV (Patent) Challenges for AVYCAZ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AVYCAZ | For Injection | avibactam sodium; ceftazidime | 0.5 g/2 g per vial | 206494 | 2 | 2024-02-26 |
US Patents and Regulatory Information for AVYCAZ
AVYCAZ is protected by ten US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AVYCAZ is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting AVYCAZ
Azabicyclic compounds, preparation thereof and use as medicines, in particular as antibacterial agents
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF TREATING BACTERIAL INFECTIONS IN COMPLICATED INTRA-ABDOMINAL INFECTION AND COMPLICATED URINARY TRACT INFECTION, INCLUDING PYELONEPHRITIS, PATIENTS COMPRISING ADMINISTERING A BACTERICIDALLY EFFECTIVE AMOUNT OF AVIBACTAM SODIUM
Azabicyclic compounds, preparation thereof and use as medicines, in particular as antibacterial agents
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF TREATING BACTERIAL INFECTIONS IN HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA AND VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA (HABP/VABP) PATIENTS COMPRISING ADMINISTERING A BACTERICIDALLY EFFECTIVE AMOUNT OF AVIBACTAM SODIUM
Azabicyclic compounds, preparation thereof and use as medicines, in particular as antibacterial agents
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING BACTERIAL INFECTIONS
Azabicyclic compounds, preparation thereof and use as medicines, in particular as antibacterial agents
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF COMPLICATED INTRA-ABDOMINAL INFECTIONS, COMPLICATED URINARY TRACT INFECTIONS, AND HOSPITAL-ACQUIRED BACTERIAL PNEUMONIA AND VENTILATOR-ASSOCIATED BACTERIAL PNEUMONIA IN ADULT AND PEDIATRIC PATIENTS (AT LEAST 31 WEEKS GESTATIONAL AGE)
Heterocyclic compounds as inhibitors of beta-lactamases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline forms of trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide sodium salt
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline forms of trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide sodium salt
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Processes for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Processes for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Processes for preparing heterocyclic compounds including trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide and salts thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting AVYCAZ
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
GENERATING ANTIBIOTIC INCENTIVES NOW
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergan | AVYCAZ | avibactam sodium; ceftazidime | POWDER;INTRAVENOUS | 206494-001 | Feb 25, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Allergan | AVYCAZ | avibactam sodium; ceftazidime | POWDER;INTRAVENOUS | 206494-001 | Feb 25, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Allergan | AVYCAZ | avibactam sodium; ceftazidime | POWDER;INTRAVENOUS | 206494-001 | Feb 25, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AVYCAZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Allergan | AVYCAZ | avibactam sodium; ceftazidime | POWDER;INTRAVENOUS | 206494-001 | Feb 25, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AVYCAZ
When does loss-of-exclusivity occur for AVYCAZ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6972
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 12270051
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2013032415
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 80403
Estimated Expiration: ⤷ Sign Up
China
Patent: 3649051
Estimated Expiration: ⤷ Sign Up
Patent: 5294690
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 21005
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 96615
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 9815
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 23800
Estimated Expiration: ⤷ Sign Up
Patent: 42462
Estimated Expiration: ⤷ Sign Up
Patent: 14517027
Estimated Expiration: ⤷ Sign Up
Patent: 17036307
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 5730
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 1020
Estimated Expiration: ⤷ Sign Up
Patent: 13014114
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 10091
Estimated Expiration: ⤷ Sign Up
Patent: 69076
Estimated Expiration: ⤷ Sign Up
Patent: 14101244
Estimated Expiration: ⤷ Sign Up
Patent: 17102358
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 5289
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2143660
Estimated Expiration: ⤷ Sign Up
Patent: 140040748
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 60404
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 65706
Estimated Expiration: ⤷ Sign Up
Patent: 1317238
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AVYCAZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2004505088 | ⤷ Sign Up | |
| European Patent Office | 2279737 | Composés hétérocycliques, actifs comme inhibiteurs de béta-lactamases (Heterocyclic compounds that are active as beta lactamase inhibitors) | ⤷ Sign Up |
| Eurasian Patent Organization | 004920 | АЗАБИЦИКЛИЧЕСКИЕ СОЕДИНЕНИЯ, СПОСОБ ИХ ПОЛУЧЕНИЯ И ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ ЛЕКАРСТВЕННЫХ СРЕДСТВ, В ЧАСТНОСТИ В КАЧЕСТВЕ АНТИБАКТЕРИАЛЬНЫХ СРЕДСТВ (AZABICYCLIC COMPOUNDS, PREPARATION THEREOF AND USE AS MEDICINES, IN PARTICULAR AS ANTIBACTERIAL AGENTS) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AVYCAZ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1480644 | 339 5029-2016 | Slovakia | ⤷ Sign Up | PRODUCT NAME: CEFTAZIDIM VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM A AVIBAKTAM VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/16/1109 20160628 |
| 1480644 | CA 2016 00059 | Denmark | ⤷ Sign Up | PRODUCT NAME: FARMACEUTISK BLANDING ELLER SAMMENSAETNING DER SOM AKTIVE BESTANDDELE DERAF, HERUNDER CEFTAZIDIMPENTAHYDRAT OG AVIBACTAMNATRIUM; REG. NO/DATE: EU/1/16/1109 20160628 |
| 1480644 | 58/2016 | Austria | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AUS CEFTAZIDIM UND AVIBACTAM; REGISTRATION NO/DATE: EU/1/16/1109 (MITTEILUNG) 20160628 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


