Last updated: December 9, 2025
Executive Summary
AVODART (dutasteride) is a prescription medication primarily used to treat benign prostatic hyperplasia (BPH) and has shown promise in other indications like male pattern baldness. As of 2023, the drug faces evolving market dynamics driven by demographic shifts, regulatory landscapes, competitive drugs, and emerging therapies. It is projected to maintain moderate growth over the next five years, influenced by patent exclusivity, healthcare policy changes, and competitive pressures. This report analyzes current market forces, revenue forecasts, competitive landscape, regulatory environment, and the financial outlook for AVODART, providing essential insights for stakeholders.
What Are the Current Market Dynamics Affecting AVODART?
1. Therapeutic Market Overview
| Indication |
Market Size (2022, USD billion) |
Key Drivers |
| Benign Prostatic Hyperplasia (BPH) |
$6.5 |
Aging male population, increasing diagnosis rates |
| Male Pattern Baldness |
$5.2 |
Rising awareness, off-label use in dermatology |
Source: IQVIA, 2022; GlobalData, 2023
2. Demographic Trends Impacting Demand
- Aging Population: The global male population aged 50+ is expected to grow by 35% between 2020 and 2030, bolstering BPH treatment needs.
- Increasing Diagnosis Rates: Greater physician awareness and improvements in diagnostic criteria expand eligible patient pools.
3. Competitive Landscape Analysis
| Competitors |
Mechanism of Action |
Market Share (2022) |
Key Features |
| Proscar (finasteride) |
5α-reductase inhibitor |
~40% |
Established, generic available |
| Tamsulosin (Flomax) |
Alpha-1 blocker |
~30% |
Symptom relief, separate class |
| Newer 5α-reductase inhibitors |
E.g., finasteride, other dutasteride formulations |
Remaining |
Patents expiring, biosimilars emerging |
Note: Dutasteride (AVODART) holds approximately 25% of the BPH market share, with revenues affected by generic entry and competitor innovation.
4. Patent and Regulatory Timeline
- Original Patent (Dutasteride): Expired in the U.S. in 2018, opening market to generics.
- Regulatory Approvals: Approved in over 80 countries; ongoing approvals for expanded indications or formulations.
Implication: Patent expiry has led to increased generic competition, reducing drug prices and profit margins.
What Are the Revenue and Financial Trajectories for AVODART?
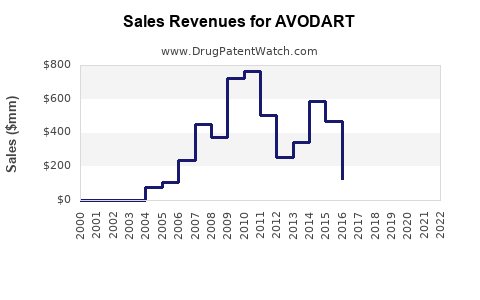
1. Revenue Trends (2018–2023)
| Year |
Global Sales (USD millions) |
Key Factors |
| 2018 |
$540 |
Patent expiry initiating generic entry |
| 2019 |
$430 |
Increased generic competition |
| 2020 |
$340 |
Pricing pressures, market saturation |
| 2021 |
$290 |
Continued generic penetration, consolidation |
| 2022 |
$250 |
Market stabilization, emergence of biosimilars |
Sources: IQVIA MAT Reports, 2023
2. Forecast for 2023–2028
| Year |
Projected Revenue (USD millions) |
Assumptions |
| 2023 |
$220 |
Market maturity, increased biosimilar competition |
| 2024 |
$200 |
Slight decline, generic proliferation continues |
| 2025 |
$180 |
Developing new indications or formulations (e.g., topical delivery) |
| 2026 |
$170 |
Potential entry of combination therapies |
| 2027 |
$160 |
Price reductions due to biosimilars, patent cliff effects |
Overall CAGR (2023–2028): Approximately -2%
Note: The trend reflects a plateau with slight decline, typical for off-patent drugs with generic competition.
What Are Strategic Opportunities and Risks?
Opportunities
- Expanded Indications: Clinical trials exploring dutasteride in hair regrowth (male pattern baldness) and other androgenic conditions could diversify revenue streams.
- Formulation Innovations: Topical or localized delivery methods could command premium pricing.
- Market Penetration in Emerging Countries: Growing healthcare access and regulatory approvals could open new markets, e.g., Asia-Pacific.
Risks
- Patent Litigation and Biosimilar Entries: Accelerate price erosion and market share loss.
- Regulatory Barriers: Stringent approval requirements, especially for new indications.
- Pricing Pressures: Payor policies favoring generics and biosimilars reduce profitability.
- Market Saturation: Limited room for growth in mature markets.
How Does AVODART Compare with Competitors?
| Parameter |
AVODART (Dutasteride) |
Finasteride (Proscar) |
Tamsulosin (Flomax) |
Emerging Drugs |
| Mechanism of Action |
5α-reductase inhibitor |
5α-reductase inhibitor |
Alpha-1 blocker |
Other pathways (e.g., PDE5 inhibitors) |
| Patent Status |
Expired (2018 in US) |
Expired |
Patent expiry pending |
Under development |
| Market Share (2022, %) |
~25% |
~40% |
~30% |
Fragmented competition |
| Typical Price (USD/tablet) |
~$2–$3 |
~$1–$2 |
~$1 |
Variable, often higher |
| Approved Indications |
BPH, off-label for baldness |
BPH, baldness |
BPH, prostate symptoms |
BPH, alopecia, others |
What Are Regulatory and Policy Trends Influencing AVODART?
1. Patent and Exclusivity Policies
- Patent Expiry: Leads to increased generic competition.
- Data Exclusivity: Typically 5–7 years in major markets; expiration accelerates commoditization.
2. Healthcare Policies
- Pricing Regulations: Countries like Canada and parts of Europe implement price caps.
- Reimbursement Strategies: Shifts towards value-based care may pressure margins for branded drugs.
3. Emerging Legislation
- Biosimilar and generic incentives: Accelerate entry and market share shifts.
- Approval pathways: Streamlining for new formulations or indications, e.g., topical dutasteride.
Comparative Analysis: Key Market Factors
| Aspect |
AVODART |
Competitor Drugs |
| Origin |
Branded (GlaxoSmithKline) |
Generic (Finasteride) or established brands |
| Price Trend |
Declining due to patent expiration |
Lower, with increasing biosimilar competition |
| Market Share |
Stable but declining |
Increasing, especially for generics |
| Regulatory Environment |
Mature, with ongoing approval for new uses |
Competitive, evolving rapidly |
Key Takeaways
- Market Maturity: AVODART faces a contracting market driven by generic displacement, with revenues declining approximately 2% annually post-2023.
- Growth Limitations: Limited prospects for growth in traditional indications; innovation and diversification are critical.
- Competitive Landscape: Dominated by first-generation 5α-reductase inhibitors; newer therapies and formulations remain on the horizon.
- Strategic Focus: Investment in expanded indications, formulation innovation, and emerging markets could provide relief from patent and pricing pressures.
- Regulatory Dynamics: Ongoing patent challenges and biosimilar entries necessitate vigilant patent management and diversification strategies.
FAQs
Q1: How has patent expiry impacted AVODART’s market share?
A: Patent expiry in the U.S. in 2018 opened markets to generics, leading to price competition and a decline from peak sales of over $540 million in 2018 to approximately $220 million in 2023.
Q2: What are the primary competitive advantages of AVODART over its rivals?
A: Its longer duration of action and clinical efficacy in reducing prostate volume distinguish it. However, brands like finasteride have broader marketing reach and lower prices.
Q3: Are there promising new indications for dutasteride?
A: Yes. Clinical trials are exploring its use in male pattern baldness, prostate cancer risk reduction, and possibly in combination therapies, which could diversify revenue sources.
Q4: What is the outlook for AVODART’s revenue in the next five years?
A: Expect a gradual decline with an approximate CAGR of -2%, stabilizing as biosimilar competition consolidates and new formulations or indications are developed.
Q5: How are regulatory policies influencing AVODART’s market?
A: Patent regulations, biosimilar pathways, and pricing policies are increasing market competition and pressure on profitability. Companies must adapt through innovation and market expansion.
Sources
- IQVIA. Medicines & Outcomes Reports, 2023.
- GlobalData. Benign Prostatic Hyperplasia Market Analysis, 2023.
- U.S. Patent and Trademark Office. Patent Status for Dutasteride, 2023.
- FDA & EMA Approvals Database, 2022–2023.
- GSK Corporate Reports, 2023.
In summary, AVODART’s future hinges on innovative strategies amidst a declining but historically significant market segment. Navigating patent expirations, enhancing indications, and tapping into emerging markets are pivotal for sustained financial performance.