AUSTEDO XR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Austedo Xr, and what generic alternatives are available?
Austedo Xr is a drug marketed by Teva and is included in one NDA. There are eleven patents protecting this drug.
This drug has one hundred and seventy-two patent family members in thirty-five countries.
The generic ingredient in AUSTEDO XR is deutetrabenazine. One supplier is listed for this compound. Additional details are available on the deutetrabenazine profile page.
DrugPatentWatch® Generic Entry Outlook for Austedo Xr
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AUSTEDO XR?
- What are the global sales for AUSTEDO XR?
- What is Average Wholesale Price for AUSTEDO XR?
Summary for AUSTEDO XR
| International Patents: | 172 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 4 |
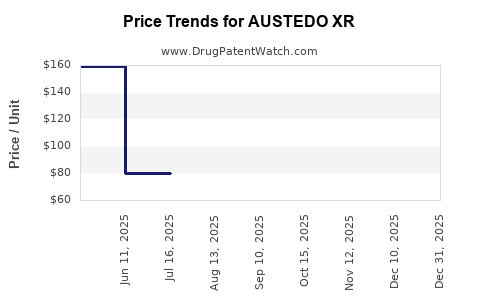
| Drug Prices: | Drug price information for AUSTEDO XR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AUSTEDO XR |
| DailyMed Link: | AUSTEDO XR at DailyMed |
Recent Clinical Trials for AUSTEDO XR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Teva Branded Pharmaceutical Products R&D, Inc. | Phase 2/Phase 3 |
| Vanderbilt University Medical Center | Phase 2/Phase 3 |
| Fundacion Huntington Puerto Rico | Phase 1 |
US Patents and Regulatory Information for AUSTEDO XR
AUSTEDO XR is protected by twelve US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva | AUSTEDO XR | deutetrabenazine | TABLET, EXTENDED RELEASE;ORAL | 216354-005 | May 29, 2024 | RX | Yes | No | 12,016,858*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Teva | AUSTEDO XR | deutetrabenazine | TABLET, EXTENDED RELEASE;ORAL | 216354-003 | Feb 17, 2023 | RX | Yes | No | 12,016,858*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Teva | AUSTEDO XR | deutetrabenazine | TABLET, EXTENDED RELEASE;ORAL | 216354-008 | Jul 1, 2024 | RX | Yes | No | 11,179,386*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Teva | AUSTEDO XR | deutetrabenazine | TABLET, EXTENDED RELEASE;ORAL | 216354-004 | May 29, 2024 | RX | Yes | No | 11,311,488 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Teva | AUSTEDO XR | deutetrabenazine | TABLET, EXTENDED RELEASE;ORAL | 216354-004 | May 29, 2024 | RX | Yes | No | 12,016,858*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for AUSTEDO XR
When does loss-of-exclusivity occur for AUSTEDO XR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13318182
Patent: Formulations pharmacokinetics of deuterated benzoquinoline inhibitors of vesicular monoamine transporter 2
Estimated Expiration: ⤷ Get Started Free
Patent: 18222896
Patent: Formulations pharmacokinetics of deuterated benzoquinoline inhibitors of vesicular monoamine transporter 2
Estimated Expiration: ⤷ Get Started Free
Patent: 20205297
Patent: Formulations pharmacokinetics of deuterated benzoquinoline inhibitors of vesicular monoamine transporter 2
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015005894
Patent: composição farmacêutica
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 83641
Patent: PHARMACOCINETIQUES DE FORMULATIONS D'INHIBITEURS DE BENZOQUINOLINE DEUTERE DU TRANSPORTEUR 2 DE MONOAMINE VESICULAIRE (FORMULATIONS PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
Patent: 24804
Patent: PHARMACOCINETIQUES DE FORMULATIONS D'INHIBITEURS DE BENZOQUINOLINE DEUTERE DU TRANSPORTEUR 2 DE MONOAMINE VESICULAIRE (FORMULATIONS PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4684555
Patent: Formulations pharmacokinetics of deuterated benzoquinoline inhibitors of vesicular monoamine transporter 2
Estimated Expiration: ⤷ Get Started Free
Patent: 1728971
Patent: D6-tetraphenylquinolizine solid oral dosage form, compound, and pharmaceutical composition, preparation method and treatment method thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 6768882
Patent: 化合物、及其药物组合物及治疗方法 (Compounds, pharmaceutical compositions and methods of treatment thereof)
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 97615
Patent: PHARMACOCINÉTIQUES DE FORMULATIONS D'INHIBITEURS DE BENZOQUINOLINE DEUTÉRÉ DU TRANSPORTEUR 2 DE MONOAMINE VÉSICULAIRE (FORMULATIONS PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 12232
Patent: 氘化苯並喹啉的囊泡單胺轉運體 抑制劑的配方藥代動力學 (FORMULATIONS PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER)
Estimated Expiration: ⤷ Get Started Free
India
Patent: 62DEN2015
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 62601
Estimated Expiration: ⤷ Get Started Free
Patent: 12420
Estimated Expiration: ⤷ Get Started Free
Patent: 15528516
Patent: 小胞モノアミン輸送体2の重水素化ベンゾキノリン阻害剤の製剤薬物動態
Estimated Expiration: ⤷ Get Started Free
Patent: 18162287
Patent: 小胞モノアミン輸送体2の重水素化ベンゾキノリン阻害剤の製剤薬物動態 (PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
Patent: 19059784
Patent: 小胞モノアミン輸送体2の重水素化ベンゾキノリン阻害剤の製剤薬物動態 (PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VASCULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
Patent: 20189871
Patent: 小胞モノアミン輸送体2の重水素化ベンゾキノリン阻害剤の製剤薬物動態 (FORMULATION PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5372
Patent: Formulations pharmacokinetics of deuterated benzoquinoline inhibitors of vesicular monoamine transporter 2
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AUSTEDO XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 114767672 | ⤷ Get Started Free | |
| Canada | 3186425 | ⤷ Get Started Free | |
| South Korea | 20250019144 | ⤷ Get Started Free | |
| European Patent Office | 2897615 | PHARMACOCINÉTIQUES DE FORMULATIONS D'INHIBITEURS DE BENZOQUINOLINE DEUTÉRÉ DU TRANSPORTEUR 2 DE MONOAMINE VÉSICULAIRE (FORMULATIONS PHARMACOKINETICS OF DEUTERATED BENZOQUINOLINE INHIBITORS OF VESICULAR MONOAMINE TRANSPORTER 2) | ⤷ Get Started Free |
| South Korea | 102528845 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: AUSTEDO XR
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
