ASPIRIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Aspirin, and what generic alternatives are available?
Aspirin is a drug marketed by Amneal Pharms, Ani Pharms, Barr, Chartwell Molecular, Dr Reddys, Glenmark Speclt, Micro Labs, Ph Health, Sandoz, Sun Pharm, and Zydus Pharms. and is included in eleven NDAs.
The generic ingredient in ASPIRIN is aspirin; dipyridamole. There are twenty-two drug master file entries for this compound. Nine suppliers are listed for this compound. Additional details are available on the aspirin; dipyridamole profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Aspirin
A generic version of ASPIRIN was approved as aspirin; dipyridamole by BARR on August 14th, 2009.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ASPIRIN?
- What are the global sales for ASPIRIN?
- What is Average Wholesale Price for ASPIRIN?
Summary for ASPIRIN
| US Patents: | 0 |
| Applicants: | 11 |
| NDAs: | 11 |
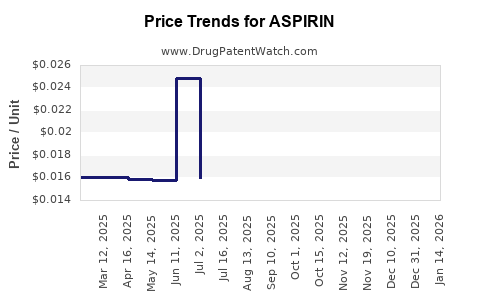
| Drug Prices: | Drug price information for ASPIRIN |
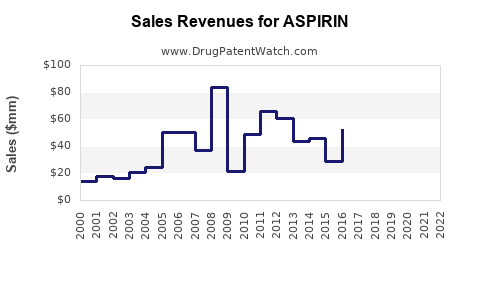
| Drug Sales Revenues: | Drug sales revenues for ASPIRIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ASPIRIN |
| DailyMed Link: | ASPIRIN at DailyMed |
Recent Clinical Trials for ASPIRIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Cincinnati | PHASE3 |
| Stanford University | PHASE3 |
| National Institute of Neurological Disorders and Stroke (NINDS) | PHASE3 |
