AMPICILLIN Drug Patent Profile
✉ Email this page to a colleague
When do Ampicillin patents expire, and what generic alternatives are available?
Ampicillin is a drug marketed by Acs Dobfar, Antibiotice, Astral, Eugia Pharma Speclts, Hikma, Hospira Inc, Hq Speclt Pharma, Istituto Bio Ita Spa, Medimetriks Pharms, Onesource Specialty, Sandoz, Acs Dobfar Spa, Apothecon, Consolidated Pharm, Hospira, Intl Medication, Lilly, Sagent Pharms Inc, Watson Labs Inc, Aurobindo Pharma, Belcher Pharms, Chartwell Rx, Ivax Sub Teva Pharms, Lederle, Mylan, Purepac Pharm, Strides Pharma Intl, Vitarine, Ph Health, and Teva. and is included in sixty-four NDAs.
The generic ingredient in AMPICILLIN is ampicillin/ampicillin trihydrate. Three suppliers are listed for this compound. Additional details are available on the ampicillin/ampicillin trihydrate profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AMPICILLIN?
- What are the global sales for AMPICILLIN?
- What is Average Wholesale Price for AMPICILLIN?
Summary for AMPICILLIN
| US Patents: | 0 |
| Applicants: | 30 |
| NDAs: | 64 |
| Drug Prices: | Drug price information for AMPICILLIN |
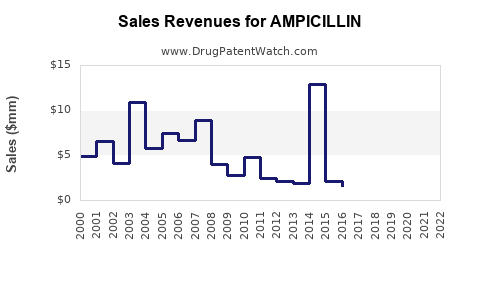
| Drug Sales Revenues: | Drug sales revenues for AMPICILLIN |
| DailyMed Link: | AMPICILLIN at DailyMed |
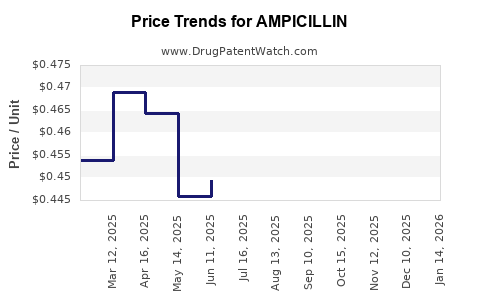
See drug prices for AMPICILLIN
US Patents and Regulatory Information for AMPICILLIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospira | AMPICILLIN SODIUM | ampicillin sodium | INJECTABLE;INJECTION | 202864-002 | Sep 4, 2015 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Sagent Pharms Inc | AMPICILLIN SODIUM | ampicillin sodium | INJECTABLE;INJECTION | 090583-001 | Nov 27, 2015 | AP | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Acs Dobfar Spa | AMPICILLIN SODIUM | ampicillin sodium | INJECTABLE;INJECTION | 090884-001 | Apr 3, 2013 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Onesource Specialty | AMPICILLIN AND SULBACTAM | ampicillin sodium; sulbactam sodium | INJECTABLE;INJECTION | 202197-001 | Apr 7, 2014 | AP | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
