ALUNBRIG Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Alunbrig, and what generic alternatives are available?
Alunbrig is a drug marketed by Takeda Pharms Usa and is included in one NDA. There are four patents protecting this drug.
This drug has ninety patent family members in thirty-nine countries.
The generic ingredient in ALUNBRIG is brigatinib. One supplier is listed for this compound. Additional details are available on the brigatinib profile page.
DrugPatentWatch® Generic Entry Outlook for Alunbrig
Alunbrig was eligible for patent challenges on April 28, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 10, 2035. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ALUNBRIG
| International Patents: | 90 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 61 |
| Clinical Trials: | 10 |
| Patent Applications: | 633 |
| Drug Prices: | Drug price information for ALUNBRIG |
| What excipients (inactive ingredients) are in ALUNBRIG? | ALUNBRIG excipients list |
| DailyMed Link: | ALUNBRIG at DailyMed |
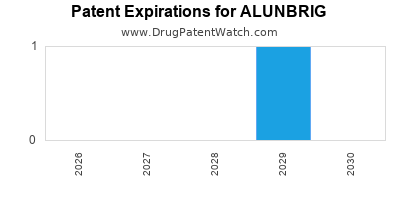

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ALUNBRIG
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ALUNBRIG
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Intergroupe Francophone de Cancerologie Thoracique | Phase 2 |
| Takeda | Phase 1/Phase 2 |
| Princess Maxima Center for Pediatric Oncology | Phase 1/Phase 2 |
Pharmacology for ALUNBRIG
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 3A Inducers Tyrosine Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ALUNBRIG
US Patents and Regulatory Information for ALUNBRIG
ALUNBRIG is protected by four US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ALUNBRIG is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ALUNBRIG
Crystalline forms of 5-chloro-N4-[-2-(dimethylphosphoryl)phenyl]-N2-{2-methoxy-4-[4-(4-methylp- iperazin-1-YL) piperidin-1-YL]phenyl}pyrimidine-2,4-diamine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC)
Phosphorous derivatives as kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Phosphorus derivatives as kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC)
Methods for inhibiting cell proliferation in ALK-driven cancers
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC)
FDA Regulatory Exclusivity protecting ALUNBRIG
TREATMENT OF PATIENTS WITH ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC) WHO HAVE PROGRESSED ON OR ARE INTOLERANT TO CRIZOTINIB
Exclusivity Expiration: ⤷ Sign Up
FOR THE TREATMENT OF ADULT PATIENTS WITH ANAPLASTIC LYMPHOMA KINASE (ALK)-POSITIVE METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC) AS DETECTED BY AN FDA-APPROVED TEST, NOT INCLUDING PATIENTS WHO HAVE PROGRESSED ON OR ARE INTOLERANT TO CRIZOTINIB
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | ALUNBRIG | brigatinib | TABLET;ORAL | 208772-001 | Apr 28, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Takeda Pharms Usa | ALUNBRIG | brigatinib | TABLET;ORAL | 208772-003 | Oct 2, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Takeda Pharms Usa | ALUNBRIG | brigatinib | TABLET;ORAL | 208772-002 | Apr 28, 2017 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ALUNBRIG
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharma A/S | Alunbrig | brigatinib | EMEA/H/C/004248 Alunbrig is indicated as monotherapy for the treatment of adult patients with anaplastic lymphoma kinase (ALK)‑positive advanced non‑small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor.Alunbrig is indicated as monotherapy for the treatment of adult patients with anaplastic lymphoma kinase ALKpositive advanced NSCLC previously treated with crizotinib. |
Authorised | no | no | no | 2018-11-22 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ALUNBRIG
When does loss-of-exclusivity occur for ALUNBRIG?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 15335950
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 65169
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 17000979
Estimated Expiration: ⤷ Sign Up
China
Patent: 7108559
Patent: 5‑氯‑N4‑[2‑(二甲基磷酰基)苯基]‑N2‑{2‑甲氧基‑4‑[4‑(4‑甲基哌嗪‑1‑基)哌啶‑1‑基]嘧啶‑2,4‑二胺的晶形 (Crystalline forms of 5-chloro-n4-[-2-(dimethylphosphoryl) phenyl]-n2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl] pyrimidine-2,4-diamine)
Estimated Expiration: ⤷ Sign Up
Patent: 1825717
Patent: 5-氯-N4-[2-(二甲基磷酰基)苯基]-N2-{2-甲氧基-4-[4-(4-甲基哌嗪-1-基)哌啶-1-基]苯基}嘧啶-2,4-二胺的晶形 (Crystalline forms of 5-chloro-N4-[-2-(dimethylphosphoryl) phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl] pyrimidine-2,4-diamine)
Estimated Expiration: ⤷ Sign Up
Patent: 1888368
Patent: 5-氯-N4-[2-(二甲基磷酰基)苯基]-N2-{2-甲氧基-4-[4-(4-甲基哌嗪-1-基)哌啶-1-基]苯基}嘧啶-2,4-二胺的晶形 (Crystalline forms of 5-chloro-N4-[-2-(dimethylphosphoryl) phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl] pyrimidine-2,4-diamine)
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 17004714
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 170146
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0201343
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 23295
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 09647
Estimated Expiration: ⤷ Sign Up
Dominican Republic
Patent: 017000101
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 17030878
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 5145
Estimated Expiration: ⤷ Sign Up
Patent: 1790892
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 09647
Estimated Expiration: ⤷ Sign Up
Patent: 60618
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 51693
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 1818
Estimated Expiration: ⤷ Sign Up
Patent: 9910
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 33072
Estimated Expiration: ⤷ Sign Up
Patent: 17535538
Estimated Expiration: ⤷ Sign Up
Patent: 20063276
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 09647
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 2216
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 17005120
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0940
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 171344
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 017500732
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 09647
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 737
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201702980Q
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 09647
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1702737
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2331856
Estimated Expiration: ⤷ Sign Up
Patent: 170072905
Estimated Expiration: ⤷ Sign Up
Patent: 210142781
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 13726
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 17000157
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 9794
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ALUNBRIG around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 2019024 | ⤷ Sign Up | |
| South Korea | 20160132127 | 키나아제 억제제로서 포스포러스 유도체 (PHOSPHOROUS DERIVATIVES AS KINASE INHIBITORS) | ⤷ Sign Up |
| Netherlands | 300990 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ALUNBRIG
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2300013 | C201930036 | Spain | ⤷ Sign Up | PRODUCT NAME: BRIGATINIB, O SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO.; NATIONAL AUTHORISATION NUMBER: EU/1/18/1264; DATE OF AUTHORISATION: 20181122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1264; DATE OF FIRST AUTHORISATION IN EEA: 20181122 |
| 2300013 | 132019000000069 | Italy | ⤷ Sign Up | PRODUCT NAME: BRIGATINIB, O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(ALUNBRIG); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1264, 20181126 |
| 2300013 | 122019000046 | Germany | ⤷ Sign Up | PRODUCT NAME: BRIGATINIB, ODER EIN PHARMAZEUTISCH UNBEDENKLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/18/1264 20181122 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


