AKLIEF Drug Patent Profile
✉ Email this page to a colleague
When do Aklief patents expire, and when can generic versions of Aklief launch?
Aklief is a drug marketed by Galderma Labs Lp and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has sixty patent family members in twenty-eight countries.
The generic ingredient in AKLIEF is trifarotene. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the trifarotene profile page.
DrugPatentWatch® Generic Entry Outlook for Aklief
Aklief was eligible for patent challenges on October 4, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 30, 2033. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for AKLIEF
| International Patents: | 60 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 33 |
| Clinical Trials: | 3 |
| Patent Applications: | 30 |
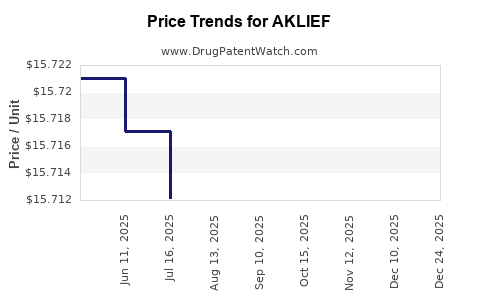
| Drug Prices: | Drug price information for AKLIEF |
| What excipients (inactive ingredients) are in AKLIEF? | AKLIEF excipients list |
| DailyMed Link: | AKLIEF at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AKLIEF
Generic Entry Date for AKLIEF*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CREAM;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for AKLIEF
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Teva Pharmaceuticals, Inc. | Phase 3 |
| Teva Pharmaceuticals USA | Phase 3 |
| Galderma R&D | Phase 4 |
Pharmacology for AKLIEF
| Drug Class | Retinoid |
Anatomical Therapeutic Chemical (ATC) Classes for AKLIEF
Paragraph IV (Patent) Challenges for AKLIEF
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AKLIEF | Cream | trifarotene | 0.005% | 211527 | 2 | 2023-10-04 |
US Patents and Regulatory Information for AKLIEF
AKLIEF is protected by five US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AKLIEF is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting AKLIEF
Ligands that modulate RAR receptors and pharmaceutical/cosmetic compositions comprised thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Ligands that modulate RAR receptors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS
Ligands that modulate RAR receptors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF ACTIVATING RARGAMMA RECEPTOR
Topical compositions containing a retinoid of the oil-in-water emulsion type
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACNE VULGARIS
Topical compositions in the form of a gel containing a particular solubilized retinoid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS
FDA Regulatory Exclusivity protecting AKLIEF
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for AKLIEF
When does loss-of-exclusivity occur for AKLIEF?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13269583
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2014029885
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 74474
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 14003226
Estimated Expiration: ⤷ Try a Trial
China
Patent: 4507469
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 54802
Estimated Expiration: ⤷ Try a Trial
France
Patent: 91177
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 04975
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6001
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 71527
Estimated Expiration: ⤷ Try a Trial
Patent: 15523342
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 4540
Estimated Expiration: ⤷ Try a Trial
Patent: 14014560
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 2472
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 37408
Estimated Expiration: ⤷ Try a Trial
Patent: 14152998
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201408759X
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1408744
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2127022
Estimated Expiration: ⤷ Try a Trial
Patent: 150028252
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 91299
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AKLIEF around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2637408 | СОДЕРЖАЩИЕ РЕТИНОИД КОМПОЗИЦИИ ДЛЯ МЕСТНОГО ПРИМЕНЕНИЯ ТИПА ЭМУЛЬСИИ "МАСЛО В ВОДЕ" (RETINOID-CONTAINING COMPOUNDS FOR LOCAL APPLICATION OF "OIL IN WATER" EMULSION TYPE) | ⤷ Try a Trial |
| China | 104507469 | Oil/water-emulsion-type topical compositions containing a retinoid | ⤷ Try a Trial |
| China | 104507471 | Topical compositions in the form of a gel containing a particular solubilised retinoid | ⤷ Try a Trial |
| Canada | 2874474 | COMPOSITIONS TOPIQUES, CONTENANT UN RETINOIDE, DE TYPE EMULSION HUILE DANS EAU (OIL/WATER-EMULSION-TYPE TOPICAL COMPOSITIONS CONTAINING A RETINOID) | ⤷ Try a Trial |
| Brazil | 112014029879 | composições tópicas em forma de gel que contêm um retinoide particular solubilizado | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AKLIEF
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1831149 | 301042 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TRIFAROTEEN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; NATIONAL REGISTRATION NO/DATE: RVG 124058 20200310; FIRST REGISTRATION: GB PL 10590/0071 20200113 |
| 1831149 | PA2022002 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: TRIFAROTENAS, PASIRINKTINAI FARMACINIU POZIURIU PRIIMTINOS DRUSKOS FORMOS ; REGISTRATION NO/DATE: PL 10590/0071 20200113 |
| 1831149 | 719 | Finland | ⤷ Try a Trial | |
| 1831149 | SPC/GB20/027 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TRIFAROTENE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK PL 10590/0071 -0001 20200113 |
| 1831149 | 132020000000088 | Italy | ⤷ Try a Trial | PRODUCT NAME: TRIFAROTENE, FACOLTATIVAMENTE NELLA FORMA DI UN SALE FARMACEUTICAMENTE ACCETTABILE(SELGAMIS); AUTHORISATION NUMBER(S) AND DATE(S): PL 10590/0071 - 0001, 20200113;047209010-022-034-046, 20200520 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


