PRASUGREL - Generic Drug Details
✉ Email this page to a colleague
Summary for PRASUGREL
| US Patents: | 0 |
| Tradenames: | 3 |
| Applicants: | 10 |
| NDAs: | 10 |
| Drug Master File Entries: | 19 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 127 |
| Clinical Trials: | 225 |
| Patent Applications: | 186 |
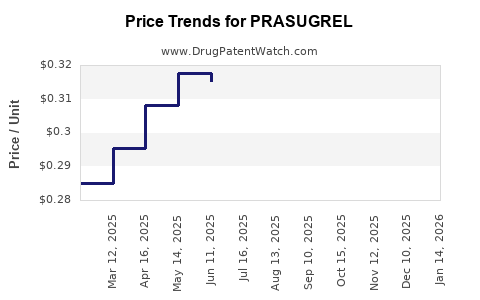
| Drug Prices: | Drug price trends for PRASUGREL |
| What excipients (inactive ingredients) are in PRASUGREL? | PRASUGREL excipients list |
| DailyMed Link: | PRASUGREL at DailyMed |
Recent Clinical Trials for PRASUGREL
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dolnolskie Centrum Chorb Serca im.prof. Zbigniewa Religi MEDINET Sp. z o.o. | PHASE3 |
| Medical Research Agency, Poland | PHASE3 |
| J.P.S Henriques | PHASE4 |
Anatomical Therapeutic Chemical (ATC) Classes for PRASUGREL
US Patents and Regulatory Information for PRASUGREL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan | PRASUGREL | prasugrel hydrochloride | TABLET;ORAL | 205927-002 | Jul 12, 2017 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Panacea | PRASUGREL | prasugrel hydrochloride | TABLET;ORAL | 205897-002 | Oct 16, 2017 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Aurobindo Pharma | PRASUGREL | prasugrel hydrochloride | TABLET;ORAL | 205888-002 | Oct 16, 2017 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for PRASUGREL
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharmaceuticals Limited | Prasugrel Mylan | prasugrel | EMEA/H/C/004644Prasugrel Mylan, co administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndrome (i.e. unstable angina, non-ST segment elevation myocardial infarction [UA/NSTEMI] or ST segment elevation myocardial infarction [STEMI]) undergoing primary or delayed percutaneous coronary intervention (PCI). | Authorised | yes | no | no | 2018-05-15 | |
| Substipharm | Efient | prasugrel | EMEA/H/C/000984Efient, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in patients with acute coronary syndrome (i.e. unstable angina, non-ST-segment-elevation myocardial infarction [UA / NSTEMI] or ST-segment-elevation myocardial infarction [STEMI]) undergoing primary or delayed percutaneous coronary intervention (PCI). | Authorised | no | no | no | 2009-02-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |