NORDITROPIN Drug Profile
✉ Email this page to a colleague
Summary for Tradename: NORDITROPIN
| High Confidence Patents: | 14 |
| Applicants: | 1 |
| BLAs: | 1 |
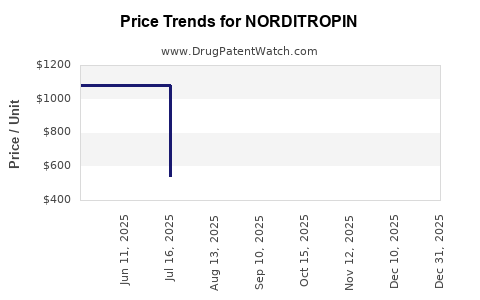
| Drug Prices: | Drug price information for NORDITROPIN |
| Recent Clinical Trials: | See clinical trials for NORDITROPIN |
Recent Clinical Trials for NORDITROPIN
Identify potential brand extensions & biosimilar entrants
| Sponsor | Phase |
|---|---|
| Shenzhen Kexing Pharmaceutical Co., Ltd. | PHASE2 |
| Xiamen Amoytop Biotech Co., Ltd. | Phase 2/Phase 3 |
| Tongji Hospital | Phase 2/Phase 3 |
Pharmacology for NORDITROPIN
| Established Pharmacologic Class | Recombinant Human Growth Hormone |
| Chemical Structure | Human Growth Hormone |
Note on Biologic Patents
Matching patents to biologic drugs is far more complicated than for small-molecule drugs.
DrugPatentWatch employs three methods to identify biologic patents:
- Brand-side disclosures in response to biosimilar applications
- DrugPatentWatch analysis and company disclosures
- Patents from broad patent text search
These patents were identified from disclosures by the brand-side company, in response to a potential biosimilar seeking to launch. They have a high certainty of blocking biosimilar entry. The expiration dates listed are not estimates — they're expiration dates as indicated by the brand-side company.
These patents were identified from searching various sources, including drug labels and other general disclosures from the brand-side company. This list may exclude some of the patents which block biosimilar launch, and some of these patents listed may not actually block biosimilar launch. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
For completeness, these patents were identified by searching the patent literature for mentions of the branded or ingredient name of the drug. Some of these patents protect the original drug, whereas others may protect follow-on inventions or even inventions casually mentioning the drug. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
1) High Certainty: US Patents for NORDITROPIN Derived from Brand-Side Litigation
No patents found based on brand-side litigation
2) High Certainty: US Patents for NORDITROPIN Derived from DrugPatentWatch Analysis and Company Disclosures
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2015-01-13 | DrugPatentWatch analysis and company disclosures |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2014-03-24 | DrugPatentWatch analysis and company disclosures |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2015-03-10 | DrugPatentWatch analysis and company disclosures |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2015-06-02 | DrugPatentWatch analysis and company disclosures |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
3) Low Certainty: US Patents for NORDITROPIN Derived from Patent Text Search
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2036-12-20 | Patent claims search |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2036-04-08 | Patent claims search |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2034-02-06 | Patent claims search |
| Novo Nordisk Inc. | NORDITROPIN | somatropin | Injection | 021148 | ⤷ Get Started Free | 2037-11-28 | Patent claims search |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
International Patents for NORDITROPIN
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| New Zealand | 246556 | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 8604609 | ⤷ Get Started Free |
| China | 1056537 | ⤷ Get Started Free |
| Hungary | P0003920 | ⤷ Get Started Free |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for NORDITROPIN
| Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|
| 2022C/513 | Belgium | ⤷ Get Started Free | PRODUCT NAME: LONAPEGSOMATROPIN; AUTHORISATION NUMBER AND DATE: EU/1/21/1607 20220117 |
| 22C1029 | France | ⤷ Get Started Free | PRODUCT NAME: LONAPEGSOMATROPINE; REGISTRATION NO/DATE: EU/1/21/1607 20220117 |
| CA 2022 00020 | Denmark | ⤷ Get Started Free | PRODUCT NAME: LONAPEGSOMATROPIN; REG. NO/DATE: EU/1/21/1607 20220117 |
| SPC/GB22/023 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: LONAPEGSOMATROPIN; REGISTERED: UK EU/1/21/1607(FOR NI) 20220117; UK FURTHER MAS ON IPSUM 20220117 |
| >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Biologic Drug: NORDITROPIN
More… ↓