Last updated: January 21, 2026
Executive Summary
Aytu Bioscience, Inc. (NASDAQ: AYTU) operates within the specialty pharmaceutical sector, focusing on urology, pediatrics, and sleep health. As of 2023, Aytu’s strategic positioning involves targeted product portfolios, a diversified revenue stream, and an evolving pipeline. This analysis evaluates Aytu’s market standing relative to key competitors, its core strengths, challenges, and strategic opportunities. It also provides data-driven insights for investors and industry stakeholders seeking a comprehensive understanding of Aytu’s positioning within the broader pharmaceutical landscape.
Market Position Overview
| Metric |
Data Point |
Source/Date |
| Market Capitalization |
Approx. $100 million (as of Q1 2023) |
NASDAQ, Q1 2023 |
| Revenue (2022) |
~$60 million |
Aytu’s Annual Report, 2022 |
| R&D Investment |
~$8 million (13% of revenue) |
Aytu’s Annual Report, 2022 |
| Key Product Focus |
Urology (e.g., Mycovisc, ZolpiMist), Sleep (e.g., Tuzistra), Pediatrics (e.g., Refonos) |
Corporate Website |
| Geo-Exposure |
Primarily North America |
Geographic filings |
Competitive Positioning
Aytu sits within a niche segment of the specialty pharmaceuticals market—focusing primarily on niche indications such as sleep disorders, urology, and pediatric health. Its competitive positioning is characterized by:
- A portfolio of FDA-approved products with some for which the company holds patent protections.
- A limited but focused pipeline, with potential for growth via new indications or formulation modifications.
- A market cap significantly below larger players like Jazz Pharmaceuticals or Horizon Therapeutics, reflecting its smaller scale and developmental stage.
Core Strengths of Aytu Bioscience
1. Portfolio of FDA-Approved Products
Aytu’s leading revenue-generating products include:
| Product Name |
Indication |
Marketed Since |
Key Attributes |
| Tuzistra XR |
Cough & cold (Extended-release) |
2017 |
Controlled-release formulation aimed at adults and pediatrics |
| ZolpiMist (Zolpidem) |
Sleep aid |
2012 |
Nasal spray delivery, rapid onset |
| Mycovisc (Hyaluronic Acid) |
Interstitial cystitis (IC) |
2020 |
Reduced pain in bladder symptoms |
| Refonos |
Pediatric constipation |
2021 |
FDA-approved for children |
2. Focus on Niche and Underserved Markets
Aytu concentrates on markets with unmet needs, such as:
- Pediatric sleep disorders
- Interstitial cystitis
- Chronic cough in adults and children
This targeted approach limits direct competition and offers opportunities to attain market share in defined segments.
3. Strategic Alliances and Distribution Channels
- Partnerships with specialty distributors enhance product reach.
- Regulatory collaborations facilitate expedited approval or expansion processes.
- In-licensing agreements extend pipeline options and diversify risk.
4. Flexibility in Product Development
Aytu’s development strategy emphasizes reformulation, delivery innovation, and expanding indications, leveraging its focused R&D investments.
Key Challenges & Risks
| Challenge/Risk |
Description |
Impact |
| Market Penetration & Awareness |
Limited brand recognition compared to larger pharma incumbents |
Slower sales growth |
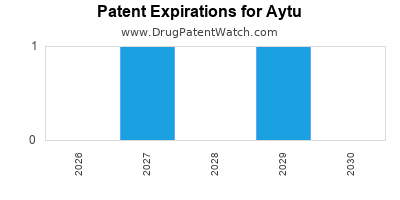
| Patent and Regulatory Risks |
Patent expiration threats; regulatory hurdles for pipeline |
Revenue erosion; delays |
| Competition from Larger Firms |
Larger companies entering niche markets with aggressive pricing |
Market share erosion |
| Limited Pipeline & R&D Budget |
Smaller R&D investment relative to peers |
Innovation slowdown |
Competitive Landscape Comparison
| Competitors |
Market Cap (USD) |
Revenue 2022 |
Product Portfolio Focus |
R&D Investment (% Revenue) |
Notable Products |
| Jazz Pharmaceuticals |
~$7.3B |
~$2.6B |
Psychiatry, Sleep, Oncology |
12-15% |
Xyrem, Sunosi |
| Horizon Therapeutics |
~$16.5B |
~$2.8B |
Rare Diseases, Autoimmune |
10-12% |
Rasilez, Krystexxa |
| American Health Packaging |
Private |
N/A |
OTC & Generic Pharma |
N/A |
Generic OTC Drugs |
| Aytu Bioscience |
~$100M |
~$60M |
Urology, Sleep, Pediatrics |
~13% |
Tuzistra, ZolpiMist, Mycovisc, Refonos |
Note: Aytu’s smaller scale limits its resources but offers agility and niche specialization.
Strategic Insights
What are Aytu’s growth prospects amidst competitive pressures?
- Pipeline Expansion: Prioritizing novel formulations and new indications in sleep and urology could widen revenue streams.
- Market Penetration: Increasing direct sales efforts and expanding distribution channels in North America.
- Business Development: Potential acquisition of complementary assets or licensing agreements to accelerate growth.
How does Aytu differentiate itself in the competitive landscape?
- Focus on Niche Indications: Less saturated segments, such as pediatric sleep and bladder health.
- FDA-Approved Portfolio: A strong foundation with proven product efficacy.
- Agile Operations: Smaller scale allows for quicker decision-making and product iterations.
What are the main strategic risks?
- Patent expirations, especially for flagship products.
- Limited R&D budget constraining innovation.
- Market entry barriers and competition from larger firms with considerable marketing budgets.
How does regulatory policy influence Aytu’s positioning?
- Favorable policies around drug repurposing and reformulation could reduce development costs.
- Stringent IP protections reinforce long-term exclusivity.
- Potential policy shifts on prescription drug pricing could compress margins.
Comparative SWOT Analysis
| Aspect |
Aytu Bioscience |
Larger Competitors |
| Strengths |
Niche focus, FDA-approved products, agility |
Broad portfolios, extensive R&D, global reach |
| Weaknesses |
Smaller scale, limited pipeline, less financial muscle |
Larger risk buffers, diversified revenues, extensive pipelines |
| Opportunities |
New indication approvals, partnerships, market expansion |
M&A, pipeline diversification, global expansion |
| Threats |
Patent expiry, competitive entries, regulatory changes |
Pricing pressures, patent cliffs, regulatory scrutiny |
Key Market Trends Impacting Aytu
- Rise of Personalized Medicine: Smaller firms can capitalize on reformulating existing drugs tailored to specific populations.
- Increased Focus on Pediatric and Sleep Health: Growing awareness raises market size for Aytu’s core products.
- Regulatory Incentives: Orphan drug designation and fast-track approvals could benefit pipeline candidates.
Conclusion and Strategic Recommendations
| Recommendation |
Rationale |
| Expand pipeline through licensing and M&A |
To offset pipeline stagnation and diversify revenue streams |
| Strengthen sales and marketing channels |
To boost market penetration in target niches |
| Invest in formulation innovation and delivery technology |
To improve product efficacy and patient adherence |
| Engage in strategic alliances with specialty clinics |
To increase prescriber adoption and patient access |
| Monitor patent landscapes and act proactively |
To extend product lifecycle and manage patent cliff risks |
Key Takeaways
- Aytu’s competitive advantage derives from its FDA-approved niche products and strategic focus on underserved markets.
- The company's smaller scale limits R&D and marketing budgets but provides agility to adapt to market changes.
- Strategic expansion, including pipeline growth and distribution enhancement, is crucial for future success.
- Increased competition and patent expirations pose significant risks; proactive patent management and pipeline innovation are critical.
- Market trends favor Aytu’s focus areas, especially with rising awareness of pediatric sleep issues and bladder health.
FAQs
-
What are Aytu's most significant revenue drivers?
Tuzistra XR and ZolpiMist account for the majority of Aytu's revenues, supported by the company's focus on cough, cold, and sleep indications.
-
How does Aytu compare to larger pharma firms in innovation?
Aytu relies primarily on reformulations of existing drugs and has a relatively limited pipeline, whereas larger firms invest heavily in novel therapies and broad R&D.
-
What opportunities exist for Aytu in the current regulatory environment?
Fast-track and orphan drug designations can expedite approvals, providing competitive advantages for pipeline products.
-
What are the main risks associated with Aytu's growth strategy?
Patent expirations, limited financial resources for R&D, and intense competition in niche markets could hinder growth.
-
How can Aytu strengthen its market position?
By expanding its pipeline, increasing marketing efforts, and forging strategic partnerships, Aytu can accelerate growth within its niche sectors.
References
- Aytu Bioscience, Inc. Annual Report 2022
- NASDAQ Listings, Q1 2023
- Industry Reports: Specialty Pharma Market Analysis, 2022-2023
- FDA Database, Product Approvals and ANDA filings, 2023