Am Regent Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and what generic alternatives to AM REGENT drugs are available?
AM REGENT has eighty approved drugs.
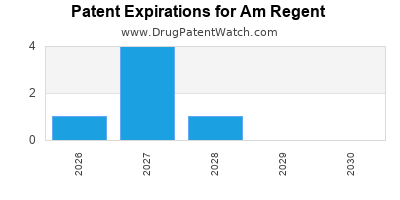
There are eight US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-four supplementary protection certificates in fifteen countries.
Drugs and US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | SELENIOUS ACID | selenious acid | SOLUTION;INTRAVENOUS | 209379-001 | Apr 30, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Am Regent | TESTOSTERONE CYPIONATE | testosterone cypionate | INJECTABLE;INJECTION | 207742-001 | Jun 16, 2017 | AO | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | RX | Yes | Yes | 7,754,702 | ⤷ Sign Up | ⤷ Sign Up | ||||
| Am Regent | CLONIDINE HYDROCHLORIDE | clonidine hydrochloride | INJECTABLE;INJECTION | 091104-001 | Oct 8, 2009 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Am Regent | STERILE WATER FOR INJECTION | sterile water for injection | LIQUID;N/A | 217341-002 | Aug 29, 2023 | AP | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-004 | Feb 4, 2022 | 11,123,321 | ⤷ Sign Up |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | 11,291,645 | ⤷ Sign Up |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | 11,590,097 | ⤷ Sign Up |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 10,519,252 | ⤷ Sign Up |
| Am Regent | DEXFERRUM | ferric oxyhydroxide | INJECTABLE;INJECTION | 040024-001 | Feb 23, 1996 | 5,624,668 | ⤷ Sign Up |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 11,590,097 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Am Regent Drugs
Supplementary Protection Certificates for Am Regent Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0933372 | PA2008006 | Lithuania | ⤷ Sign Up | PRODUCT NAME: FOSAMPRENAVIR CALCIUM; REGISTRATION NO/DATE: EU/1/04/282/001-002 20040712 |
| 1554315 | C01554315/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: EISEN ALS EISENCARBOXYMALTOSE; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 57851 10.11.2022 |
| 1499331 | SPC/GB13/034 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: IZINOVA CONCENTRATE FOR ORAL SOLUTION. THE ACTIVE SUBSTANCE IS A MIXTURE OF 3 SALTS:SODIUM SULPHATE ANHYDROUS, MAGNESIUM SULPHATE HEPTAHYDRATE AND POTASSIUM SULPHATE.; REGISTERED: BE BE434323 20130220; UK PL34926/0016 20130313 |
| 0145340 | 99C0005 | Belgium | ⤷ Sign Up | PRODUCT NAME: FOSPHENYTOIN DISODIUM; NAT. REGISTRATION NO/DATE: NL 23 613 19980806; FIRST REGISTRATION: GB - PL 000 19/0157 19980204 |
| 0771217 | CA 2006 00038 | Denmark | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETA-CYCLODEXTRIN-CLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 38687 20060627; FIRST REG. NO/DATE: EU RVG 31781 20050804 |
| 1718641 | 2012/008 | Ireland | ⤷ Sign Up | PRODUCT NAME: AZILSARTAN MEDOXOMIL AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING THE POTASSIUM SALT; REGISTRATION NO/DATE: EU/1/11/734/001-011 EU/1/11/735/001-011 20111209 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.