Listen to this article |
DrugPatentWatch has deep resources to support patent prosecution and patent litigation, but it is also used by law firms for business development.
Table of Contents
Find weak claims
 One of the first tweaks requested by a law firm was the ability to run a broad search against drug patent claims. The rationale for this request is that an attorney had recognized that a recent court ruling could be applied to any claims containing specific language. By searching for this language across all drug patents she felt she could identify patents at risk for invalidity.
One of the first tweaks requested by a law firm was the ability to run a broad search against drug patent claims. The rationale for this request is that an attorney had recognized that a recent court ruling could be applied to any claims containing specific language. By searching for this language across all drug patents she felt she could identify patents at risk for invalidity.
This search can be used by brand-side and generic-side attorneys:
- Brand-side patent counsel can identify patents held by their clients that are at risk for Paragraph IV patent challenges and proactively engage in strategies to mitigate these risks
- Generic-side patent counsel can identify weak drug patents and help their clients be first to file Paragraph IV patent challenges and possibly win first-challenger 180-day generic exclusivity
Which drugs will face patent challenges, and when?
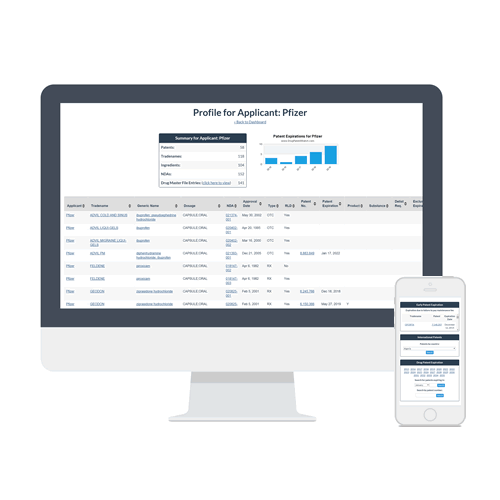
 An easy way for brand-side attorneys to expand their practice is to identify drugs facing patent challenges and prepare to defend these patents or to expand the patent portfolio. DrugPatentWatch provides one-click access to drugs facing near-term litigation through the New Chemical Entity Exclusivity (NCE) dates.
An easy way for brand-side attorneys to expand their practice is to identify drugs facing patent challenges and prepare to defend these patents or to expand the patent portfolio. DrugPatentWatch provides one-click access to drugs facing near-term litigation through the New Chemical Entity Exclusivity (NCE) dates.
Because the FDA will not accept generic drug applications — with or without patent challenges — until one-year before NCE expiration, you can use DrugPatentWatch’s list of NCE expirations to track which drugs are future candidates for patent challenges.
- Generic-side attorneys can use these lists to identify patent challenge opportunities and advise their clients on which drugs they may want to consider adding to their generic portfolios.
- Brand-side attorneys can use these lists to proactively contact existing clients and develop strategies to prepare for impending patent litigation. They can also be used to identify potential new clients.
When can generics launch?
Many generic drug companies opt to avoid patent challenges. Instead of aggressively seeking early entry, it is also possible to wait until patents expire, or for other challengers to challenge patents, and to enter only when market-entry barriers have been removed.
Two ways in which lawyers can provide inexpensive guidance as part of a long-term client-acquisition strategy are to share information on expiring 180-day patent challenge exclusivity, or to share estimated generic launch opportunity dates.
 The first company to successfully challenge and defeat a drug patent is eligible for 180-days of generic market exclusivity. Patent challenge can be expensive and risky, so some generic companies prefer to wait until first-generics have entered, and then launch only after these first-entrants have exhausted their generic market exclusivity. Sharing these second-entrant launch opportunities can build affinity with growing generic companies, helping secure future legal representation opportunities.
The first company to successfully challenge and defeat a drug patent is eligible for 180-days of generic market exclusivity. Patent challenge can be expensive and risky, so some generic companies prefer to wait until first-generics have entered, and then launch only after these first-entrants have exhausted their generic market exclusivity. Sharing these second-entrant launch opportunities can build affinity with growing generic companies, helping secure future legal representation opportunities.
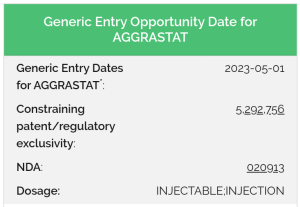 Aside from tracking invalidated patents, it can also be useful to track anticipated generic entry dates. While parsing the myriad patents protecting a drug can be expensive, attorneys can offer a less-expensive option for clients with limited budgets.
Aside from tracking invalidated patents, it can also be useful to track anticipated generic entry dates. While parsing the myriad patents protecting a drug can be expensive, attorneys can offer a less-expensive option for clients with limited budgets.
By using DrugPatentWatch’s estimates of generic entry opportunities lawyers can accommodate clients with limited budgets, in anticipation of larger projects.
Who is trying to work around patents?
It may be possible to work around drug patents by modifying the active ingredient, changing the formulation, or making other changes and filing what is called a 505(b)(2) hybrid drug application. DrugPatentWatch has extensive lists of 505(b)(2) clinical trials for drugs, making it simple to identify companies developing modified versions of drugs — well before they file for FDA approval.
 Brand-side and generic-side attorneys alike can use these lists for business development.
Brand-side and generic-side attorneys alike can use these lists for business development.
- Brand-side attorneys can use the 505(b)(2) lists to identify potential new entrants. If these companies may be infringing on patents it may be possible to file injunctions and stop them before launch and if not then branded companies can prepare for 505(b)(2) entry.
- Generic-side attorneys can identify future 505(b)(2) entrants and help them ensure that they are steering clear of patents and further help them develop patent portfolios around their modified versions of drugs.
Drug name synonyms for prior-art searches
 Drugs can have many names in the course of their development. In addition to the development names being used by companies seeking marketing approvals, unaffiliated research scientists can also publish papers and may file patents based on the chemical names associated with drugs. Any published document mentioning a drug — by any of its names — can be used to either as prior art (to invalidate patents) or to pursue infringement claims against generic entrants.
Drugs can have many names in the course of their development. In addition to the development names being used by companies seeking marketing approvals, unaffiliated research scientists can also publish papers and may file patents based on the chemical names associated with drugs. Any published document mentioning a drug — by any of its names — can be used to either as prior art (to invalidate patents) or to pursue infringement claims against generic entrants.
DrugPatentWatch publishes exhaustive lists of drug synonyms, arming you with the information you need to ensure the completeness of your prior art searches.
Conclusion
DrugPatentWatch is used directly by branded and generic drug companies, and by attorneys to support their clients. Over time we have seen an increased sharing of usage between business development and client-facing functions. This article presented some of the more common ways in which law firms have used DrugPatentWatch to support brand-side or generic-side business development.