Cream dosed drug patent expirations by year
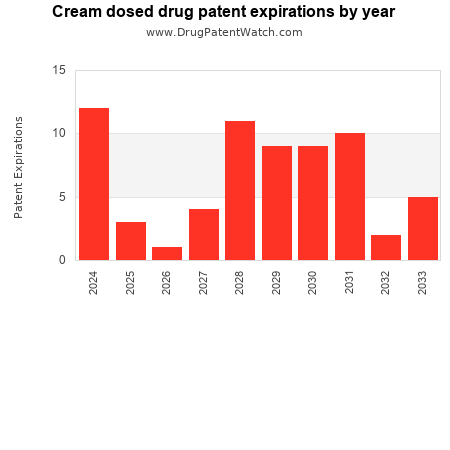
This chart shows the patent expirations for cream dosed drugs over the next decade.
The term of drug patents varies. The basic term for a patent is 20 years from the date of patent filing, which generally occurs several years before a drug is approved. This means that drugs may have 6-12 years of patent-protected sales after launch. There are conditions under which drug patents can be extended, for example to compensate for time spent waiting for Food and Drug Agency (FDA) review, or for responding to an FDA request for pediatric testing.
Drug patent expirations can also be used as a bellwether for the pharmaceutical industry — a rise, or fall, in the number of anticipated patent expirations can predict outcomes for branded pharmceutical companies, generic producers, and for healthcare payers.
The long-range forecast presented here can help model pharmaceutical industry trends for cream dosed drugs over the coming decade.


