zulresso Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Zulresso, and when can generic versions of Zulresso launch?
Zulresso is a drug marketed by Sage Therap and is included in one NDA. There are eight patents protecting this drug.
This drug has one hundred and nineteen patent family members in thirty-one countries.
The generic ingredient in ZULRESSO is brexanolone. One supplier is listed for this compound. Additional details are available on the brexanolone profile page.
DrugPatentWatch® Generic Entry Outlook for Zulresso
Zulresso was eligible for patent challenges on June 17, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 13, 2029. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for zulresso
| International Patents: | 119 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 56 |
| Clinical Trials: | 7 |
| Patent Applications: | 585 |
| Drug Prices: | Drug price information for zulresso |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for zulresso |
| What excipients (inactive ingredients) are in zulresso? | zulresso excipients list |
| DailyMed Link: | zulresso at DailyMed |
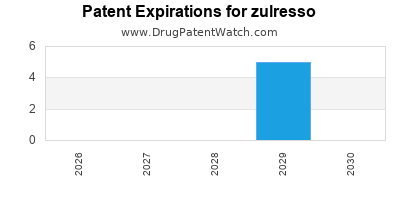

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for zulresso
Generic Entry Date for zulresso*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for zulresso
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Brigham and Women's Hospital | Phase 4 |
| Sage Therapeutics | Phase 1 |
| Donald Jeffrey Newport | Phase 4 |
Anatomical Therapeutic Chemical (ATC) Classes for zulresso
US Patents and Regulatory Information for zulresso
zulresso is protected by eight US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of zulresso is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting zulresso
Sulfoalkyl ether cyclodextrin compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Anticonvulsant activity of steroids
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING POSTPARTUM DEPRESSION
Neuroactive steroid formulations and methods of treating CNS disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Neuroactive steroids, compositions, and uses thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING POSTPARTUM DEPRESSION
Sulfoalkyl ether cyclodextrin compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Sulfoalkyl ether cyclodextrin compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Sulfoalkyl ether cyclodextrin compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Sulfoalkyl ether cyclodextrin compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting zulresso
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sage Therap | ZULRESSO | brexanolone | SOLUTION;INTRAVENOUS | 211371-001 | Jun 17, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for zulresso
When does loss-of-exclusivity occur for zulresso?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09241858
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0905080
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 02603
Estimated Expiration: ⤷ Try a Trial
Patent: 71879
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1959508
Estimated Expiration: ⤷ Try a Trial
Patent: 5288650
Estimated Expiration: ⤷ Try a Trial
Patent: 4053423
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1355
Estimated Expiration: ⤷ Try a Trial
Patent: 1000828
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 68269
Estimated Expiration: ⤷ Try a Trial
Patent: 63430
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 19883
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 8956
Estimated Expiration: ⤷ Try a Trial
Patent: 3654
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 23144
Estimated Expiration: ⤷ Try a Trial
Patent: 39721
Estimated Expiration: ⤷ Try a Trial
Patent: 76828
Estimated Expiration: ⤷ Try a Trial
Patent: 44548
Estimated Expiration: ⤷ Try a Trial
Patent: 10539193
Estimated Expiration: ⤷ Try a Trial
Patent: 12072160
Estimated Expiration: ⤷ Try a Trial
Patent: 15110671
Estimated Expiration: ⤷ Try a Trial
Patent: 17019879
Estimated Expiration: ⤷ Try a Trial
Patent: 18052991
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 2459
Estimated Expiration: ⤷ Try a Trial
Patent: 10004900
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 9290
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1314803
Estimated Expiration: ⤷ Try a Trial
Patent: 110010742
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering zulresso around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 112472814 | 神经活性类固醇制剂和治疗中枢神经系统障碍的方法 (Neuroactive steroid formulations and methods of treating CNS disorders) | ⤷ Try a Trial |
| Japan | 2021152074 | ステロイドの抗痙攣活性 (ANTICONVULSANT ACTIVITY OF STEROIDS) | ⤷ Try a Trial |
| European Patent Office | 3650027 | FORMULATIONS STÉROÏDIENNES NEUROACTIVES COMPRENANT UN COMPLEXE D'ALLOPRÉGNANOLONE ET D'ÉTHER SULFOBUTYLIQUE BÊTA-CYCLODEXTRINE (NEUROACTIVE STEROID FORMULATIONS COMPRISING A COMPLEX OF ALLOPREGNANOLONE AND SULFOBUTYL ETHER BETA-CYCLODEXTRIN) | ⤷ Try a Trial |
| Japan | 6444548 | ⤷ Try a Trial | |
| New Zealand | 627781 | Neuroactive steroid formulations and methods of treating cns disorders | ⤷ Try a Trial |
| Philippines | 12018501923 | NEUROACTIVE STEROIDS, COMPOSITIONS, AND USES THEREOF | ⤷ Try a Trial |
| South Korea | 20180115797 | 신경활성 스테로이드, 조성물, 및 그의 용도 | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


