XPHOZAH Drug Patent Profile
✉ Email this page to a colleague
When do Xphozah patents expire, and when can generic versions of Xphozah launch?
Xphozah is a drug marketed by Ardelyx Inc and is included in one NDA. There are five patents protecting this drug.
This drug has ninety-two patent family members in twenty-nine countries.
The generic ingredient in XPHOZAH is tenapanor hydrochloride. One supplier is listed for this compound. Additional details are available on the tenapanor hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Xphozah
Xphozah was eligible for patent challenges on September 12, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 1, 2033. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for XPHOZAH?
- What are the global sales for XPHOZAH?
- What is Average Wholesale Price for XPHOZAH?
Summary for XPHOZAH
| International Patents: | 92 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Drug Prices: | Drug price information for XPHOZAH |
| DailyMed Link: | XPHOZAH at DailyMed |
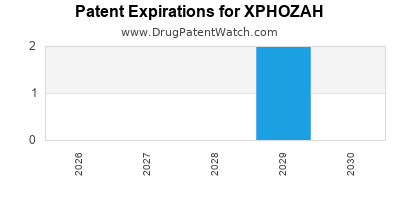

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for XPHOZAH
Generic Entry Date for XPHOZAH*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for XPHOZAH
US Patents and Regulatory Information for XPHOZAH
XPHOZAH is protected by seven US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of XPHOZAH is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,541,448.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ardelyx Inc | XPHOZAH | tenapanor hydrochloride | TABLET;ORAL | 213931-001 | Oct 17, 2023 | DISCN | Yes | No | 10,940,146 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ardelyx Inc | XPHOZAH | tenapanor hydrochloride | TABLET;ORAL | 213931-003 | Oct 17, 2023 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ardelyx Inc | XPHOZAH | tenapanor hydrochloride | TABLET;ORAL | 213931-001 | Oct 17, 2023 | DISCN | Yes | No | 8,541,448 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for XPHOZAH
When does loss-of-exclusivity occur for XPHOZAH?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09334511
Patent: Compounds and methods for inhibiting NHE-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0923861
Patent: Compostos e métodos para inibir o antiporte nhe-mediado no tratamento de distúrbios associados com a retenção de líquidos ou com a sobrecarga de sal e distúrbios do trato gastrointestinal.
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 48607
Patent: COMPOSES ET PROCEDES D'INHIBITION D'UN ANTIPORT A MEDIATION PAR NHE DANS LE TRAITEMENT DE TROUBLES ASSOCIES A UNE RETENTION DE FLUIDE OU A UNE SURCHARGE DE SEL ET DE TROUBLES DU T RACTUS GASTRO-INTESTINAL (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2333759
Patent: Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
Estimated Expiration: ⤷ Get Started Free
Patent: 3819403
Patent: Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180289
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20451
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 84318
Patent: COMPOSÉS ET PROCÉDÉS D'INHIBITION D'UN ANTIPORT À MÉDIATION PAR NHE DANS LE TRAITEMENT DE TROUBLES ASSOCIÉS À UNE RÉTENTION DE FLUIDE OU À UNE SURCHARGE DE SEL ET DE TROUBLES DU TRACTUS GASTRO-INTESTINAL (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 51248
Patent: COMPOSÉS ET PROCÉDÉS D'INHIBITION D'UN ANTIPORT À MÉDIATION PAR NHE DANS LE TRAITEMENT DE TROUBLES ASSOCIÉS À UNE RÉTENTION DE FLUIDE OU À UNE SURCHARGE DE SEL ET DE TROUBLES DU TRACTUS GASTRO-INTESTINAL (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 39964
Patent: COMBINAISONS D'INHIBITION D'UN ANTIPORT A MEDIATION PAR NHE DANS LE TRAITEMENT DE TROUBLES ASSOCIES A UNE RETENTION DE FLUIDE OU A UNE SURCHARGE DE SEL ET DE TROUBLES DU TRACTUS GASTRO-INTESTINAL (COMBINATIONS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 98162
Patent: 用於治療與體液瀦留或鹽超負荷有關的病症和胃腸道病症的化合物和方法 (COMPOUNDS AND METHODS IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 36405
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3852
Patent: תרכובות ושיטות לעיכוב אנטיפורט מתווך nhe בטיפול בהפרעות הקשורות לאצירת נוזלים או עומס יתר של מלחים ואי-סדרים במערכת העיכול (Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 0641
Patent: תכשירי רוקחות לעיכוב אנטיפורט מתווך nhe בטיפול בהפרעות הקשורות לאצירת נוזלים או עומס יתר של מלחים ואי-סדרים במערכת העיכול (Pharmaceutical compositions for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders)
Estimated Expiration: ⤷ Get Started Free
Patent: 9851
Patent: תרכובות ושיטות לעיכוב אנטיפורט מתווך nhe בטיפול בהפרעות הקשורות לאצירת נוזלים או עומס יתר של מלחים ואי-סדרים במערכת העיכול (Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 02106
Estimated Expiration: ⤷ Get Started Free
Patent: 05802
Estimated Expiration: ⤷ Get Started Free
Patent: 12514009
Estimated Expiration: ⤷ Get Started Free
Patent: 14114300
Patent: COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 5283
Patent: COMPUESTOS Y METODOS PARA INHIBIR EL ANTIPORTE MEDIADO POR INTERCAMBIADOR DE IONES DE SODIO/IONES DE HIDROGENO (NHE) EN EL TRATAMIENTO DE TRASTORNOS ASOCIADOS CON RETENCION DE FLUIDO O SOBRECARGA DE SAL Y TRASTORNOS DEL TRACTO GASTROINTESTINAL. (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 1407
Patent: COMPUESTOS Y MÉTODOS PARA INHIBIR EL ANTIPORTE MEDIADO POR INTERCAMBIADOR DE IONES DE SODIO/IONES DE HIDRÓGENO (NHE) EN EL TRATAMIENTO DE TRASTORNOS ASOCIADOS CON RETENCIÓN DE FLUIDO O SOBRECARGA DE SAL Y TRASTORNOS DEL TRACTO GASTROINTESTINAL. (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 11007024
Patent: COMPUESTOS Y METODOS PARA INHIBIR EL ANTIPORTE MEDIADO POR INTERCAMBIADOR DE IONES DE SODIO/IONES DE HIDROGENO (NHE) EN EL TRATAMIENTO DE TRASTORNOS ASOCIADOS CON RETENCION DE FLUIDO O SOBRECARGA DE SAL Y TRASTORNOS DEL TRACTO GASTROINTESTINAL. (COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS.)
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01800071
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 84318
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1683318
Estimated Expiration: ⤷ Get Started Free
Patent: 1766619
Estimated Expiration: ⤷ Get Started Free
Patent: 110110287
Patent: COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS
Estimated Expiration: ⤷ Get Started Free
Patent: 160140994
Patent: 체액 저류 또는 염 과부하와 연관된 장애 및 위장관 장애의 치료 시에 NHE-매개된 역수송을 억제하는 화합물 및 방법 (- COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 170091783
Patent: 체액 저류 또는 염 과부하와 연관된 장애 및 위장관 장애의 치료 시에 NHE-매개된 역수송을 억제하는 화합물 및 방법 (- COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 200111230
Patent: 체액 저류 또는 염 과부하와 연관된 장애 및 위장관 장애의 치료 시에 NHE-매개된 역수송을 억제하는 화합물 및 방법 (- COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 220042487
Patent: 체액 저류 또는 염 과부하와 연관된 장애 및 위장관 장애의 치료 시에 NHE-매개된 역수송을 억제하는 화합물 및 방법 (- COMPOUNDS AND METHODS FOR INHIBITING NHE-MEDIATED ANTIPORT IN THE TREATMENT OF DISORDERS ASSOCIATED WITH FLUID RETENTION OR SALT OVERLOAD AND GASTROINTESTINAL TRACT DISORDERS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 57938
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering XPHOZAH around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2018168165 | ⤷ Get Started Free | |
| Taiwan | 201446246 | ⤷ Get Started Free | |
| South Korea | 20150140386 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: XPHOZAH
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
