XEPI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Xepi, and when can generic versions of Xepi launch?
Xepi is a drug marketed by Ferrer Internacional and is included in one NDA. There are two patents protecting this drug.
This drug has thirty-seven patent family members in twenty-three countries.
The generic ingredient in XEPI is ozenoxacin. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the ozenoxacin profile page.
DrugPatentWatch® Generic Entry Outlook for Xepi
Xepi was eligible for patent challenges on December 11, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 29, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for XEPI
| International Patents: | 37 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 53 |
| Patent Applications: | 58 |
| What excipients (inactive ingredients) are in XEPI? | XEPI excipients list |
| DailyMed Link: | XEPI at DailyMed |
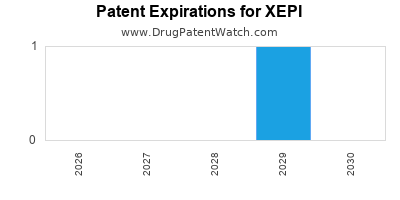
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for XEPI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for XEPI
| Drug Class | Quinolone Antimicrobial |
Anatomical Therapeutic Chemical (ATC) Classes for XEPI
US Patents and Regulatory Information for XEPI
XEPI is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of XEPI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting XEPI
Pharmaceutical topical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF IMPETIGO DUE TO STAPHYLOCOCCUS AUREUS OR STREPTOCOCCUS PYOGENES
Pharmaceutical topical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF IMPETIGO DUE TO STAPHYLOCOCCUS AUREUS OR STREPTOCOCCUS PYOGENES
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferrer Internacional | XEPI | ozenoxacin | CREAM;TOPICAL | 208945-001 | Dec 11, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Ferrer Internacional | XEPI | ozenoxacin | CREAM;TOPICAL | 208945-001 | Dec 11, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for XEPI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ferrer Internacional | XEPI | ozenoxacin | CREAM;TOPICAL | 208945-001 | Dec 11, 2017 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for XEPI
When does loss-of-exclusivity occur for XEPI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3901
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 09305360
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0920355
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 38384
Estimated Expiration: ⤷ Sign Up
Patent: 22167
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 11000848
Estimated Expiration: ⤷ Sign Up
Patent: 15002899
Estimated Expiration: ⤷ Sign Up
China
Patent: 2186460
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 14838
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 44130
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 77208
Estimated Expiration: ⤷ Sign Up
Patent: 44130
Estimated Expiration: ⤷ Sign Up
Patent: 70117
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 61552
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 09209
Estimated Expiration: ⤷ Sign Up
Patent: 73120
Estimated Expiration: ⤷ Sign Up
Patent: 12505867
Estimated Expiration: ⤷ Sign Up
Patent: 14088456
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 8029
Estimated Expiration: ⤷ Sign Up
Patent: 11004052
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 0884
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 014502425
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 44130
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 44130
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 78367
Estimated Expiration: ⤷ Sign Up
Patent: 11119764
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 44130
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1682256
Estimated Expiration: ⤷ Sign Up
Patent: 110071086
Estimated Expiration: ⤷ Sign Up
Patent: 160025034
Estimated Expiration: ⤷ Sign Up
Patent: 160116353
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 37967
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 04418
Estimated Expiration: ⤷ Sign Up
Patent: 1018497
Estimated Expiration: ⤷ Sign Up
Patent: 1511779
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 187
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering XEPI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Uruguay | 32187 | COMPOSICIONES FARMACEUTICAS TOPICAS | ⤷ Sign Up |
| Cyprus | 1114838 | ⤷ Sign Up | |
| Russian Federation | 2478367 | СТАБИЛЬНАЯ КОМПОЗИЦИЯ (ВАРИАНТЫ), ЛЕКАРСТВЕННОЕ СРЕДСТВО (ВАРИАНТЫ) И СПОСОБЫ ЛЕЧЕНИЯ И ПРОФИЛАКТИКИ КОЖНЫХ ИНФЕКЦИЙ И ИНФЕКЦИЙ КОЖНЫХ СТРУКТУР, ВЫЗВАННЫХ БАКТЕРИЯМИ, ЗАБОЛЕВАНИЙ, ПЕРЕДАЮЩИХСЯ ПОЛОВЫМ ПУТЕМ, И ИНФЕКЦИЙ ПОЛОВЫХ ПУТЕЙ, ВЫЗВАННЫХ БАКТЕРИЯМИ, И ИНФЕКЦИЙ НОСОГЛОТКИ, ВЫЗВАННЫХ БАКТЕРИЯМИ, У ЧЕЛОВЕКА ИЛИ ЖИВОТНОГО (STABLE COMPOSITION (VERSIONS), MEDICATION (VERSIONS) AND METHODS OF TREATMENT AND PREVENTION OF SKIN INFECTIONS AND INFECTIONS OF SKIN STRUCTURES, CAUSED BY BACTERIA, SEXUALLY TRANSMITTED DISEASES AND INFECTIONS OF REPRODUCTIVE TRACT, CAUSED BY BACTERIA, NASOPHARYNGEAL INFECTIONS, CAUSED BY BACTERIA, IN HUMANS OR ANIMALS) | ⤷ Sign Up |
| Mexico | 338029 | COMPOSICIONES FARMACEUTICAS TOPICAS. (PHARMACEUTICAL TOPICAL COMPOSITIONS.) | ⤷ Sign Up |
| Russian Federation | 2011119764 | СТАБИЛЬНАЯ КОМПОЗИЦИЯ (ВАРИАНТЫ), ЛЕКАРСТВЕННОЕ СРЕДСТВО (ВАРИАНТЫ) И СПОСОБЫ ЛЕЧЕНИЯ И ПРОФИЛАКТИКИ КОЖНЫХ ИНФЕКЦИЙ И ИНФЕКЦИЙ КОЖНЫХ СТРУКТУР, ЗАБОЛЕВАНИЙ, ПЕРЕДАЮЩИХСЯ ПОЛОВЫМ ПУТЕМ И ИНФЕКЦИЙ ПОЛОВЫХ ПУТЕЙ, ИНФЕКЦИЙ НОСОГЛОТКИ У ЧЕЛОВЕКА ИЛИ ЖИВОТНОГО | ⤷ Sign Up |
| South Korea | 101682256 | ⤷ Sign Up | |
| Portugal | 2344130 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XEPI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2344130 | 132017000093887 | Italy | ⤷ Sign Up | PRODUCT NAME: OZENOXACIN(DUBINE); AUTHORISATION NUMBER(S) AND DATE(S): ES/H/414/01/DC, 20170519;045237017, 20170711 |
| 2344130 | 2017C/031 | Belgium | ⤷ Sign Up | PRODUCT NAME: OZENOXACINE; AUTHORISATION NUMBER AND DATE: BE509591 20170519 |
| 2344130 | 300884 | Netherlands | ⤷ Sign Up | PRODUCT NAME: OZENOXACIN; REGISTRATION NO/DATE: ES/H/414/1/DC 20170519 |
| 2344130 | 2017/038 | Ireland | ⤷ Sign Up | PRODUCT NAME: OZENOXACIN; NAT REGISTRATION NO/DATE: PA1744/003/001 20170616; FIRST REGISTRATION NO/DATE: ES/H/414/01/DC 20170519 |
| 2344130 | C201730036 | Spain | ⤷ Sign Up | PRODUCT NAME: OZENOXACINO; NATIONAL AUTHORISATION NUMBER: 82357-ES/H/0414/001/DC; DATE OF AUTHORISATION: 20170830; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): ES/H/414/01/DC; DATE OF FIRST AUTHORISATION IN EEA: 20170519 |
| 2344130 | 1790031-7 | Sweden | ⤷ Sign Up | PRODUCT NAME: OZENOXACIN; NAT. REG. NO/DATE: 54608 20170622; FIRST REG.: BE ES/H/414/01/DC 20170519 |
| 2344130 | 122017000073 | Germany | ⤷ Sign Up | PRODUCT NAME: OZENOXACIN; NAT. REGISTRATION NO/DATE: 97303.00.00 20170803; FIRST REGISTRATION: BELGIEN BE509591 20170517 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


