TUDORZA PRESSAIR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tudorza Pressair, and when can generic versions of Tudorza Pressair launch?
Tudorza Pressair is a drug marketed by Covis and is included in one NDA. There are two patents protecting this drug.
This drug has seventy-two patent family members in thirty-six countries.
The generic ingredient in TUDORZA PRESSAIR is aclidinium bromide. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the aclidinium bromide profile page.
DrugPatentWatch® Generic Entry Outlook for Tudorza Pressair
Tudorza Pressair was eligible for patent challenges on July 23, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 13, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TUDORZA PRESSAIR?
- What are the global sales for TUDORZA PRESSAIR?
- What is Average Wholesale Price for TUDORZA PRESSAIR?
Summary for TUDORZA PRESSAIR
| International Patents: | 72 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Patent Applications: | 484 |
| Drug Prices: | Drug price information for TUDORZA PRESSAIR |
| What excipients (inactive ingredients) are in TUDORZA PRESSAIR? | TUDORZA PRESSAIR excipients list |
| DailyMed Link: | TUDORZA PRESSAIR at DailyMed |
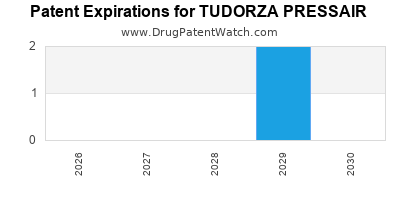

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TUDORZA PRESSAIR
Generic Entry Date for TUDORZA PRESSAIR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER, METERED;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for TUDORZA PRESSAIR
| Drug Class | Anticholinergic |
| Mechanism of Action | Cholinergic Antagonists |
US Patents and Regulatory Information for TUDORZA PRESSAIR
TUDORZA PRESSAIR is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TUDORZA PRESSAIR is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covis | TUDORZA PRESSAIR | aclidinium bromide | POWDER, METERED;INHALATION | 202450-001 | Jul 23, 2012 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Covis | TUDORZA PRESSAIR | aclidinium bromide | POWDER, METERED;INHALATION | 202450-001 | Jul 23, 2012 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TUDORZA PRESSAIR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Covis | TUDORZA PRESSAIR | aclidinium bromide | POWDER, METERED;INHALATION | 202450-001 | Jul 23, 2012 | ⤷ Get Started Free | ⤷ Get Started Free |
| Covis | TUDORZA PRESSAIR | aclidinium bromide | POWDER, METERED;INHALATION | 202450-001 | Jul 23, 2012 | ⤷ Get Started Free | ⤷ Get Started Free |
| Covis | TUDORZA PRESSAIR | aclidinium bromide | POWDER, METERED;INHALATION | 202450-001 | Jul 23, 2012 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for TUDORZA PRESSAIR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Covis Pharma Europe B.V. | Bretaris Genuair | aclidinium bromide | EMEA/H/C/002706Bretaris Genuair is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). | Authorised | no | no | no | 2012-07-20 | |
| Covis Pharma Europe B.V. | Eklira Genuair | aclidinium bromide | EMEA/H/C/002211Eklira Genuair is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). | Authorised | no | no | no | 2012-07-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TUDORZA PRESSAIR
When does loss-of-exclusivity occur for TUDORZA PRESSAIR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0835
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09224895
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0905775
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 16724
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 09000602
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2083416
Estimated Expiration: ⤷ Get Started Free
Patent: 4473911
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 90636
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0151214
Estimated Expiration: ⤷ Get Started Free
Patent: 0220919
Estimated Expiration: ⤷ Get Started Free
Patent: 0220929
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16926
Estimated Expiration: ⤷ Get Started Free
Patent: 25381
Estimated Expiration: ⤷ Get Started Free
Patent: 25382
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010300
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 00599
Estimated Expiration: ⤷ Get Started Free
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54890
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 45815
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27726
Estimated Expiration: ⤷ Get Started Free
Patent: 59019
Estimated Expiration: ⤷ Get Started Free
Patent: 59020
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1132
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 70460
Estimated Expiration: ⤷ Get Started Free
Patent: 11513451
Estimated Expiration: ⤷ Get Started Free
Patent: 14139233
Estimated Expiration: ⤷ Get Started Free
Patent: 16130248
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7027
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 8774
Estimated Expiration: ⤷ Get Started Free
Patent: 10008235
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 286
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5857
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 091672
Estimated Expiration: ⤷ Get Started Free
Patent: 141036
Estimated Expiration: ⤷ Get Started Free
Patent: 190406
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 08713
Estimated Expiration: ⤷ Get Started Free
Patent: 10141333
Estimated Expiration: ⤷ Get Started Free
Patent: 14140674
Estimated Expiration: ⤷ Get Started Free
Patent: 19100425
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 241
Estimated Expiration: ⤷ Get Started Free
Patent: 398
Estimated Expiration: ⤷ Get Started Free
Patent: 399
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8825
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 65257
Estimated Expiration: ⤷ Get Started Free
Patent: 54889
Estimated Expiration: ⤷ Get Started Free
Patent: 54891
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1003900
Patent: NOVEL DOSAGE AND FORMULATION
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 100126322
Estimated Expiration: ⤷ Get Started Free
Patent: 180125055
Estimated Expiration: ⤷ Get Started Free
Patent: 200054329
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 51307
Estimated Expiration: ⤷ Get Started Free
Patent: 14674
Estimated Expiration: ⤷ Get Started Free
Patent: 16831
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 39296
Estimated Expiration: ⤷ Get Started Free
Patent: 0938232
Patent: Novel dosage and formulation
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1652
Patent: КОМПОЗИЦИЯ ДЛЯ ИНГАЛЯЦИИ, КОТОРАЯ СОДЕРЖИТ АКЛИДИНИЙ ДЛЯ ЛЕЧЕНИЯ АСТМЫ И ХРОНИЧЕСКОГО ОБСТРУКТИВНОГО ЗАБОЛЕВАНИЯ ЛЕГКИХ;КОМПОЗИЦІЯ ДЛЯ ІНГАЛЯЦІЇ, ЩО МІСТИТЬ АКЛІДИНІЙ ДЛЯ ЛІКУВАННЯ АСТМИ ТА ХРОНІЧНОГО ОБСТРУКТИВНОГО ЗАХВОРЮВАННЯ ЛЕГЕНЬ (COMPOSITION FOR INHALATION COMPRISING ACLIDINIUM FOR THE TREATMENT OF ASTHMA AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 687
Patent: NUEVA DOSIFICACION Y FORMULACION
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TUDORZA PRESSAIR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Argentina | 002534 | CARTUCHO PARA UN POLVO FARMACEUTICO Y UN INHALADOR QUE LO COMPRENDE | ⤷ Get Started Free |
| China | 1325129 | ⤷ Get Started Free | |
| Uruguay | 26244 | NUEVOS DERIVADOS DE QUINUCLIDINA Y COMPOSICIONES FARMACÉUTICAS QUE LOS CONTIENEN. | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TUDORZA PRESSAIR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1200431 | 2013/002 | Ireland | ⤷ Get Started Free | PRODUCT NAME: ACLIDINIUM SALT WITH A PHARMACEUTICALLY ACCEPTABLE ANION OF A MONO OR POLYVALENT ACID ESPECIALLY AS ACLIDINIUM BROMIDE; NAT REGISTRATION NO/DATE: EU/1/12/778/001-003 20120720; FIRST REGISTRATION NO/DATE: EU/1/12/781/001-003 20/07/2012 EUROPEAN UNION EU/1/12/778/001-003 20/07/2012 EUROPEAN UNION EU/1/12/781/001-003 20120720 |
| 1200431 | C01200431/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: ACLIDINIUM; REGISTRATION NO/DATE: SWISSMEDIC 62590 25.04.2013 |
| 1200431 | PA2013001,C1200431 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: ACLIDINII BROMIDUM; REGISTRATION NO/DATE: EU/1/12/778/001 - EU/1/12/778/003, 2012 07 20 EU/1/12/781/001 - EU/1/12/781/003 20120720 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TUDORZA PRESSAIR (Aclidinium Bromide)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
