TERBINAFINE Drug Patent Profile
✉ Email this page to a colleague
When do Terbinafine patents expire, and when can generic versions of Terbinafine launch?
Terbinafine is a drug marketed by Taro, Aurobindo Pharma, Breckenridge Pharm, Chartwell, Cipla, Dr Reddys Labs Inc, Emed Medcl, Gedeon Richter Usa, Glenmark Generics, Heritage Pharma Avet, Invagen Pharms, Mylan, Orbion Pharms, Roxane, and Wockhardt. and is included in sixteen NDAs.
The generic ingredient in TERBINAFINE is terbinafine hydrochloride. There are twenty-seven drug master file entries for this compound. Forty-one suppliers are listed for this compound. Additional details are available on the terbinafine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Terbinafine
A generic version of TERBINAFINE was approved as terbinafine hydrochloride by TARO on July 2nd, 2007.
Summary for TERBINAFINE
| US Patents: | 0 |
| Applicants: | 15 |
| NDAs: | 16 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for TERBINAFINE |
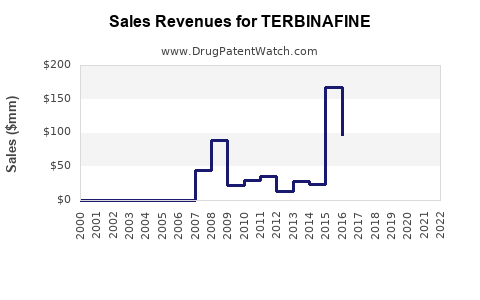
| Drug Sales Revenues: | Drug sales revenues for TERBINAFINE |
| DailyMed Link: | TERBINAFINE at DailyMed |
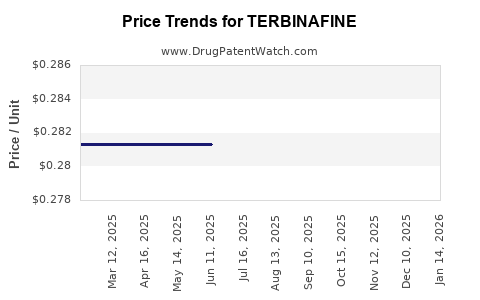
See drug prices for TERBINAFINE
Recent Clinical Trials for TERBINAFINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dhaka Medical College | Phase 2/Phase 3 |
| IQVIA Biotech | Phase 3 |
| Moberg Pharma AB | Phase 3 |


