TEKAMLO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tekamlo, and what generic alternatives are available?
Tekamlo is a drug marketed by Novartis and is included in one NDA. There is one patent protecting this drug.
This drug has twenty-three patent family members in twenty-one countries.
The generic ingredient in TEKAMLO is aliskiren hemifumarate; amlodipine besylate. There are four drug master file entries for this compound. Additional details are available on the aliskiren hemifumarate; amlodipine besylate profile page.
DrugPatentWatch® Generic Entry Outlook for Tekamlo
Tekamlo was eligible for patent challenges on March 5, 2011.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 21, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TEKAMLO?
- What are the global sales for TEKAMLO?
- What is Average Wholesale Price for TEKAMLO?
Summary for TEKAMLO
| International Patents: | 23 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Patent Applications: | 31 |
| DailyMed Link: | TEKAMLO at DailyMed |
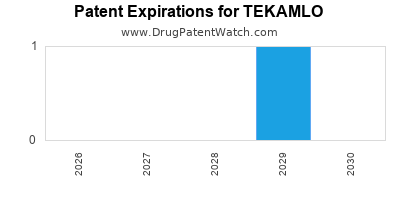
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TEKAMLO
Generic Entry Date for TEKAMLO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
US Patents and Regulatory Information for TEKAMLO
TEKAMLO is protected by one US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TEKAMLO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-001 | Aug 26, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-004 | Aug 26, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-002 | Aug 26, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-003 | Aug 26, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TEKAMLO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-001 | Aug 26, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-004 | Aug 26, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-003 | Aug 26, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | TEKAMLO | aliskiren hemifumarate; amlodipine besylate | TABLET;ORAL | 022545-002 | Aug 26, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TEKAMLO
When does loss-of-exclusivity occur for TEKAMLO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3384
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09292908
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0919350
Patent: combinação de dose fixa oral farmacêutica na forma de uma comprimido monocamada, bem como seu uso e seu método de preparação
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 36257
Patent: FORMULATIONS GALENIQUES DE COMPOSES ORGANIQUES (GALENICAL FORMULATIONS OF ORGANIC COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11000594
Patent: Combinacion farmaceutica que comprende: a) 10-45% de alisquireno, b) 0,5-5% de amlodipina, c) 2-15% de un desintegrante, d) 1-60% de un diluyente, e) 0,1-20% de un aglutinante, f) 0,1-5% de un lubricante, g) 0,05-5% de un derrapante y h) opcionalmente un relleno; metodo de preparacion; uso para tratar hipertension.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2159195
Patent: Dual-layer or monolayer form fixed dose combination of aliskiren and amlodipine
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 51711
Patent: COMBINACION DE DOSIS FIJA EN LA FORMA DE UNA TABLETA DE DOS COPAS O DE UNA SOLA CAPA DE ALISQUIRENO Y AMLODIPINA
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11010999
Patent: COMBINACIÓN DE DOSIS FIJA EN LA FORMA DE UNA TABLETA DE DOS CAPAS O DE UNA SOLA CAPA DE ALISQUIRENO Y AMLODIPINA
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 28564
Patent: FORMULATIONS GALÉNIQUES DE COMPOSÉS ORGANIQUES (FIXED DOSE COMBINATION IN FORM OF A BILAYERED OR MONOLAYERED TABLET OF ALISKIREN AND AMLODIPINE)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 12503020
Estimated Expiration: ⤷ Get Started Free
Patent: 15091830
Patent: 有機化合物のガレヌス製剤 (GALENICAL FORMULATIONS OF ORGANIC COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 39
Patent: تركيبات جالينية من مركبات عضوية (Galenical Formulations of Organic Compounds)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3610
Patent: FIXED DOSE COMBINATION IN FORM OF A BILAYERED OR MONOLAYERED TABLET OF ALISKIREN AND AMLODIPINE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11002988
Patent: COMBINACION DE DOSIS FIJA EN LA FORMA DE UNA TABLETA DE DOS CAPAS O DE UNA SOLA CAPA DE ALISQUIRENO Y AMLODIPINA. (FIXED DOSE COMBINATION IN FORM OF A BILAYERED OR MONOLAYERED TABLET OF ALISKIREN AND AMLODIPINE.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 719
Patent: تركيبات خاصة بصناعة الأدوية من مركبات عضوية
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 110293
Patent: COMBINACION DE DOSIS FIJA EN LA FORMA DE UNA TABLETA DE UNA SOLA CAPA QUE COMPRENDE ALISQUIRENO Y AMLODIPINA
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 11115712
Patent: КОМБИНАЦИИ С ФИКСИРОВАННОЙ ДОЗОЙ АЛИСКИРЕНА И АМЛОДИПИНА В ФОРМЕ ДВУХСЛОЙНЫХ ИЛИ ОДНОСЛОЙНЫХ ТАБЛЕТОК
Estimated Expiration: ⤷ Get Started Free
Patent: 14140552
Patent: КОМБИНАЦИИ С ФИКСИРОВАННОЙ ДОЗОЙ АЛИСКИРЕНА И АМЛОДИПИНА В ФОРМЕ ДВУХСЛОЙНЫХ ИЛИ ОДНОСЛОЙНЫХ ТАБЛЕТОК
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1101644
Patent: FIXED DOSE COMBINATION IN FORM OF A BILAYERED OR MONOLAYERED TABLET OF ALISKIREN AND AMLODIPINE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 110060942
Patent: FIXED DOSE COMBINATION IN FORM OF BILAYERED OR MONOLAYERED TABLET OF ALISKIREN AND AMLODIPINE
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 1016217
Patent: Galenical formulations of organic compounds
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 11000100
Patent: GALENICAL FORMULATIONS OF ORGANIC COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TEKAMLO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 310410 | ⤷ Get Started Free | |
| Norway | 2009011 | ⤷ Get Started Free | |
| China | 1266118 | ⤷ Get Started Free | |
| Netherlands | 300386 | ⤷ Get Started Free | |
| Cyprus | 2208 | Delta-amino-gamma-hydroxy-omega-aryl alkanoic acidamides with enzyme especially renin inhibiting ac tivities | ⤷ Get Started Free |
| New Zealand | 270938 | ALPHA-AMINOALKANOIC ACIDS AND DERIVATIVES THEREOF AS REAGENTS | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TEKAMLO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0678503 | 91373 | Luxembourg | ⤷ Get Started Free | 91373, EXPIRES: 20200407 |
| 0678503 | SPC041/2007 | Ireland | ⤷ Get Started Free | SPC041/2007: 20080416, EXPIRES: 20200406 |
| 0678503 | C300386 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE OMVATTENDE ALISKIREN, ALS VRIJE BASE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN HYDROCHLOORTHIAZIDE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT ERVAN; NATL. REGISTRATION NO/DATE: EU/1/08/491/001-080 20090116; FIRST REGISTRATION: CH 58935 01-04 20081028 |
| 1915993 | 1390055-0 | Sweden | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION INNEFATTANDE ALISKIREN, ELLER ETT FARMACEUTISKT ACCEPTABELT SALT DAERAV, OCH AMLODIPIN, ELLER ETT FARMACEUTISKT ACCEPTABELT SALT DAERAV.; REG. NO/DATE: EU/1/11/686/001 20110414 |
| 0678503 | C300296 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ALISKIREN OF EEN; REGISTRATION NO/DATE: EU/1/07/405/001-020 20070822 |
| 2305232 | 122019000098 | Germany | ⤷ Get Started Free | PRODUCT NAME: ALISKIREN HEMIFUMARAT UND HYDROCHLOROTHIAZID; REGISTRATION NO/DATE: EU/1/08/491/001-080 20090116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory of TEKAMLO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
