PRESTALIA Drug Patent Profile
✉ Email this page to a colleague
When do Prestalia patents expire, and what generic alternatives are available?
Prestalia is a drug marketed by Adhera and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has thirty patent family members in twenty-seven countries.
The generic ingredient in PRESTALIA is amlodipine besylate; perindopril arginine. There are fifty drug master file entries for this compound. Additional details are available on the amlodipine besylate; perindopril arginine profile page.
DrugPatentWatch® Generic Entry Outlook for Prestalia
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 5, 2029. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PRESTALIA?
- What are the global sales for PRESTALIA?
- What is Average Wholesale Price for PRESTALIA?
Summary for PRESTALIA
| International Patents: | 30 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Patent Applications: | 2 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PRESTALIA |
| What excipients (inactive ingredients) are in PRESTALIA? | PRESTALIA excipients list |
| DailyMed Link: | PRESTALIA at DailyMed |
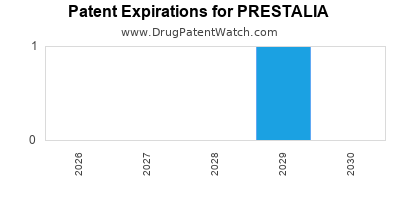
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PRESTALIA
Generic Entry Date for PRESTALIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Paragraph IV (Patent) Challenges for PRESTALIA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PRESTALIA | Tablets | amlodipine besylate; perindopril arginine | 3.5 mg/2.5 mg, 7 mg/5 mg and 14 mg/10 mg | 205003 | 1 | 2016-11-04 |
US Patents and Regulatory Information for PRESTALIA
PRESTALIA is protected by one US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PRESTALIA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-001 | Jan 21, 2015 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-002 | Jan 21, 2015 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-003 | Jan 21, 2015 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PRESTALIA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-001 | Jan 21, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-002 | Jan 21, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| Adhera | PRESTALIA | amlodipine besylate; perindopril arginine | TABLET;ORAL | 205003-003 | Jan 21, 2015 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PRESTALIA
When does loss-of-exclusivity occur for PRESTALIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07220435
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0708278
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 44467
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1389603
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160644
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 17753
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 89182
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 4716
Estimated Expiration: ⤷ Get Started Free
Patent: 0801777
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 89182
Estimated Expiration: ⤷ Get Started Free
France
Patent: 97866
Patent: FORME CRISTALLINE ALPHA DU SEL D'ARGININE DU PERINDOPRIL, SON PROCEDE DE PREPARATION, ET LES COMPOSITIONS PHARMACEUTIQUES QUI LA CONTIENNENT (New alpha crystalline form of perindopril arginine salt are angiotensin I converting enzyme inhibitor, useful for the manufacture of drugs to treat cardiovascular diseases)
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0125433
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 29669
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27898
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 55454
Estimated Expiration: ⤷ Get Started Free
Patent: 09534295
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 1035
Patent: ? CRYSTALLINE FORM OF THE ARGININE SALT OF PERINDOPRIL, A PROCESS FOR ITS PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING IT
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 456
Patent: KRISTALNI OBLIK ARGININSKE SOLI PERINDOPRILA, POSTUPAK NJENE PROIZVODNJE I FARMACEUTSKE SMEŠE KOJE JE SADRŽE (CRYSTALLINE FORM OF THE ARGININE SALT OF PERINDOPRIL, PROCESS FOR PREPARING IT, AND PHARMACEUTICAL COMPOSITIONS COMPRISING IT)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 276
Patent: FORME CRISTALLINE ALPHA DU SEL D'ARGININE DU PERINDOPRIL, SON PROCEDE DE PREPARATION, ET LES COMPOSITIONS PHARMACEUTIQUES QUI LA CONTIENNENT
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0367
Patent: A crystalline form of the arginine salt of perindopril, process for preparing it, and pharmaceutical compositions comprising it
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 083535
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 89182
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 754
Patent: KRISTALNI OBLIK ARGININSKE SOLI PERINDOPRILA, POSTUPAK NJENE PROIZVODNJE I FARMACEUTSKE SMEŠE KOJE JE SADRŽE (CRYSTALLINE FORM OF THE ARGININE SALT OF PERINDOPRIL, PROCESS FOR PREPARING IT, AND PHARMACEUTICAL COMPOSITIONS COMPRISING IT)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 89182
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0807024
Patent: Alpha crystaline form of the arginine salt of perindopril, process for preparing it, and pharmaceutical compositions comprising it
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 080106948
Patent: ALPHA; CRYSTALLINE FORM OF THE ARGININE SALT OF PERINDOPRIL, PROCESS FOR PREPARING IT, AND PHARMACEUTICAL COMPOSITIONS COMPRISING IT
Estimated Expiration: ⤷ Get Started Free
Patent: 120001818
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 81982
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 905
Patent: α КРИСТАЛЛИЧЕСКАЯ ФОРМА АРГИНИНОВОЙ СОЛИ ПЕРИНДОПРИЛА, СПОСОБ ЕЕ ПОЛУЧЕНИЯ И ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, КОТОРАЯ ЕЕ СОДЕРЖИТ;α-КРИСТАЛІЧНА ФОРМА АРГІНІНОВОЇ СОЛІ ПЕРИНДОПРИЛУ, СПОСІБ ЇЇ ОДЕРЖАННЯ І ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ, ЯКА ЇЇ МІСТИТЬ (α-CRYSTALLINE FORM OF PERINDOPRIL ARGININE SALT, METHOD FOR MAKING SAME, AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAME)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PRESTALIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1354873 | Sel de périndopril et les compositions pharmaceutiques qui le contiennent (Salt of perindopril and pharmaceutical compositions containing it) | ⤷ Get Started Free |
| Ecuador | SP045441 | NUEVA SAL DE PERINDOPRIL Y COMPOSICIONES FARMACÉUTICAS QUE LO CONTIENEN | ⤷ Get Started Free |
| New Zealand | 524478 | Arginine salt of perindopril ((2S)-2-[(1S)-carbethoxybutylamino]-1-oxo-propyl-(2S, 3aS, 7aS)perhydroindole-carboxylic acid) and pharmaceutical compositions containing it | ⤷ Get Started Free |
| Slovenia | 1989182 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 200300334 | НОВАЯ СОЛЬ ПЕРИНДОПРИЛА И СОДЕРЖАЩИЕ ЕЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ | ⤷ Get Started Free |
| Montenegro | 00454 | NOVA SO PERINDOPRILA I FARMACEUTSKE KOMPOZICIJE KOJE SADRŽE TU SO (NOVEL PERINDOPRIL SALT AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAME) | ⤷ Get Started Free |
| Eurasian Patent Organization | 005676 | НОВАЯ СОЛЬ ПЕРИНДОПРИЛА И СОДЕРЖАЩИЕ ЕЕ ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ (SALT OF PERINDOPRIL AND PHARMACEUTICAL COMPOSITIONS CONTAINING IT) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PRESTALIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1915993 | C300625 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE BEVATTENDE ALISKIREN, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN AMLODIPINE, OF EEN FARMACEUATISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/11/686/001-056 20110414 |
| 1915993 | 92315 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: COMBINAISON COMPRENANT ALISKIREN,OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLE,ET AMLODIPINE,OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLE |
| 0502314 | C300478 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: TELMISARTAN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, EN AMLODIPINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER AMLODIPINEBESILAAT; REGISTRATION NO/DATE: EU/1/10/648/001-028 20101007 |
| 0678503 | C300499 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE OMVATTEND ALISKIREN OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN AMLODIPINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/11/686/001-056 20110114 |
| 0443983 | C300445 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: VALSARTAN, AMLODIPINE EN HYDROCHLOORTHIAZIDE EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; REGISTRATION NO/DATE: EU/1/09/569/001-060 20091016 |
| 1507558 | 2012/018 | Ireland | ⤷ Get Started Free | PRODUCT NAME: ALISKIREN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AMLODIPINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; NAT REGISTRATION NO/DATE: EU/1/11/730/001-060 20111122; FIRST REGISTRATION NO/DATE: SWITZERLAND 6167801-6167805 20110705 |
| 1507558 | 12C0033 | France | ⤷ Get Started Free | PRODUCT NAME: ALISKIRENE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE E CELUI-CI, AMLODIPINE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI, ET HYDROCHLOROTHIAZIDE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; NAT. REGISTRATION NO/DATE: EU/1/11/730/001 20111122; FIRST REGISTRATION: CH - 6167801 20110705 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Prestalia
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
