Last updated: July 30, 2025
Introduction
Prednisone Intensol is a highly potent corticosteroid prescribed primarily for inflammatory and autoimmune conditions. With its active ingredient, prednisone, the drug plays a pivotal role in managing diseases such as rheumatoid arthritis, asthma, and allergic reactions. The evolving landscape of the pharmaceutical industry, driven by regulatory shifts, market demands, and innovation in corticosteroid therapies, significantly influences Prednisone Intensol's market dynamics and financial prospects. This analysis delves into the current market landscape, factors shaping its trajectory, and the key economic indicators influencing its future.
Market Overview
As of 2023, corticosteroids occupy a substantial segment of the global anti-inflammatory therapeutics market. Prednisone, being a widely used corticosteroid, generated significant revenues—driven by its broad application scope, cost-effectiveness, and established efficacy. Prednisone Intensol, a liquid formulation, aims at enhancing bioavailability and patient compliance, especially among pediatric and geriatric populations.
The drug's market share is buoyed by its generic status, with several pharmaceutical companies producing competition-rich formulations. However, branded variants typically command premium pricing due to perceived quality and reliability. The global prescription corticosteroids market is valued at approximately USD 7.2 billion (2022) with a projected compound annual growth rate (CAGR) of around 4% through 2030, influenced by rising prevalence of chronic inflammatory diseases and expanding healthcare access.
Market Drivers
1. Increasing Prevalence of Autoimmune and Chronic Inflammatory Diseases
Epidemiological data indicate a surge in autoimmune conditions such as rheumatoid arthritis, lupus, and inflammatory bowel disease. According to the Global Burden of Disease Study, autoimmune diseases affect over 5% of the global population, creating sustained demand for corticosteroids. Prednisone Intensol benefits from this trend due to its efficacy, especially in acute exacerbations.
2. Growing Geriatric Population
Aging populations worldwide face increased incidences of osteoporosis and inflammatory disorders requiring corticosteroid therapy. The WHO estimates that the proportion of people aged 60 and above will reach 16% by 2030, amplifying demand for corticosteroids like Prednisone Intensol, especially considering its suitability for chronic management.
3. Advantages of Liquid Formulations
Prednisone Intensol's liquid form enhances absorption rates compared to traditional tablets, making it preferable for pediatric, elderly, and patients with dysphagia. This advantage fuels its adoption in specialized therapeutic settings, contributing positively to sales growth.
4. Expanding Pharmaceutical Manufacturing & Generic Competition
Generic manufacturing has led to significant price reductions, facilitating broader access and usage. Competition among manufacturers also incentivizes quality improvements, further solidifying Prednisone Intensol's market position.
Market Challenges
1. Safety and Regulatory Concerns
Long-term corticosteroid use entails risks like osteoporosis, adrenal suppression, and metabolic disturbances. Regulatory agencies emphasize monitoring of adverse effects, influencing prescribing patterns and market growth.
2. Price Competition and Biosimilar Entry
The influx of biosimilars and more targeted therapies threatens traditional corticosteroid sales. While biosimilars are more prominent in biologic drugs, the emerging generic corticosteroid landscape exerts downward pressure on pricing and margins.
3. Regulatory Variability
Divergent regulatory standards across regions influence market access. Stringent approval processes in regions like the EU and North America potentially delay new formulations or therapeutic indications, impacting revenue timelines.
4. Competition from Newer Therapeutics
Biologic agents targeting specific inflammatory pathways (e.g., TNF inhibitors) increasingly replace corticosteroids in certain indications, especially in rheumatoid arthritis, reducing prednisone’s share in some segments.
Financial Trajectory
Revenue Streams and Pricing Trends
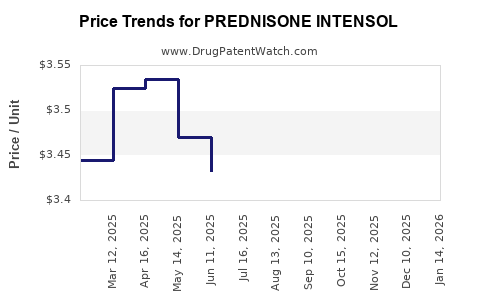
Prednisone Intensol's revenue depends heavily on generic sales, with prices declining as competition intensifies. Average wholesale prices (AWP) for corticosteroid formulations have decreased approximately 10-15% annually over the past five years, primarily due to generic penetration.
Market Penetration and Geographic Expansion
The drug maintains substantial market penetration in North America and Europe. Emerging markets like Asia-Pacific, Latin America, and Africa present growth opportunities owing to increasing healthcare infrastructure, rising disease awareness, and expanding insurance coverage.
Impact of Patent Expiry and Generic Entry
Patent expiry for various corticosteroid formulations, although Prednisone Intensol itself is generically available, influences profitability. Manufacturers often rely on incremental innovation or formulation improvements to sustain revenue streams, a trend expected to continue.
Future Revenue Forecasts
Projections estimate a CAGR of 3-5% for Prednisone Intensol's market segment over the next decade, driven by growing disease prevalence and regional market expansion. However, margins may tighten due to pricing pressures and increasing competition.
Regulatory and Innovation Trends
Regulatory agencies are prioritizing drug safety, pushing for stricter labeling and monitoring that could influence prescribing patterns. Innovative formulations—such as extended-release or targeted delivery systems—may reinvigorate demand, albeit with higher R&D costs.
In addition, the development of corticosteroids with fewer side effects and more targeted action remains a focal area, potentially impacting Prednisone Intensol’s market relevance. Companies investing in such innovation might command higher premiums or market exclusivity, altering the financial landscape.
Strategic Outlook
To navigate the evolving market dynamics, pharmaceutical companies must prioritize:
- Diversification of formulations to include targeted or sustained-release variants.
- Expanding into emerging markets via strategic partnerships and localization.
- Investing in safety and efficacy research to address regulatory concerns and foster prescriber confidence.
- Building brand recognition for quality assurance amidst generic proliferation.
- Monitoring alternative therapies including biologics and immunomodulators, which could disrupt corticosteroid demand.
Key Takeaways
- Rising disease prevalence, aging populations, and formulation advantages sustain Prednisone Intensol's demand amid competitive pressures.
- Market growth is tempered by regulatory scrutiny, safety concerns, and competitive innovations—necessitating strategic R&D investments.
- Regional expansion, especially in emerging markets, offers significant revenue opportunities amid slower growth in mature markets.
- Pricing pressures and biosimilar entrants pose risks to profit margins, urging manufacturers to differentiate through quality and innovation.
- Regulatory vigilance and proactive safety management are critical to maintaining market authorization and prescriber trust.
FAQs
1. What are the primary therapeutic uses of Prednisone Intensol?
Prednisone Intensol is indicated for inflammatory and autoimmune conditions such as rheumatoid arthritis, allergic reactions, asthma exacerbations, and certain dermatologic disorders. Its liquid form enhances absorption, especially in pediatric and geriatric patients.
2. How does Prednisone Intensol compare to other corticosteroid formulations?
Its liquid formulation offers superior bioavailability and ease of dose adjustment, particularly beneficial for patients with swallowing difficulties or requiring precise dosing. However, it faces price competition from generic tablets and other corticosteroid forms.
3. What factors could influence Prednisone Intensol’s market growth in the next decade?
Key factors include increasing chronic disease prevalence, expanding healthcare access in emerging markets, regulatory changes, competition from biosimilars and newer agents, and formulation innovation.
4. Are there upcoming regulatory or safety challenges for Prednisone Intensol?
Yes, regulators emphasize monitoring side effects like osteoporosis and metabolic disturbances. Innovations aimed at reducing adverse effects may become necessary to sustain market confidence and approval.
5. What strategic moves should pharmaceutical companies consider to sustain Prednisone Intensol’s market position?
Companies should invest in formulation innovations, expand geographic reach, focus on safety profiles, develop targeted delivery systems, and educate healthcare providers about product benefits to differentiate amidst commodification.
References
[1] Market Research Future, "Corticosteroids Market Analysis," 2022.
[2] World Health Organization, "Global Disease Burden," 2021.
[3] IQVIA, "Global Pharmaceutical Market Trends," 2022.
[4] FDA, "Guidelines on Corticosteroid Use and Safety," 2022.
[5] GlobalData, "Emerging Market Opportunities for Corticosteroids," 2023.