LOXAPINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Loxapine, and when can generic versions of Loxapine launch?
Loxapine is a drug marketed by Elite Labs Inc, Lannett Co Inc, Rising, and Watson Labs. and is included in seven NDAs.
The generic ingredient in LOXAPINE is loxapine succinate. There are eight drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the loxapine succinate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Loxapine
A generic version of LOXAPINE was approved as loxapine succinate by WATSON LABS on June 15th, 1988.
Summary for LOXAPINE
| US Patents: | 0 |
| Applicants: | 4 |
| NDAs: | 7 |
| Formulation / Manufacturing: | see details |
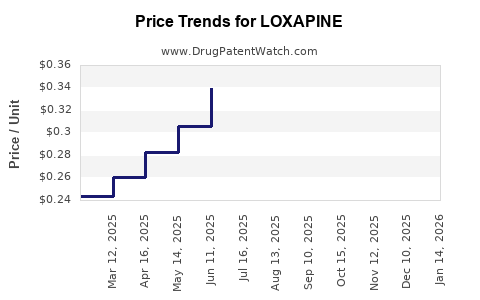
| Drug Prices: | Drug price information for LOXAPINE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LOXAPINE |
| DailyMed Link: | LOXAPINE at DailyMed |
Recent Clinical Trials for LOXAPINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Assistance Publique - Hôpitaux de Paris | Phase 3 |
| Lariboisière-Saint Louis clinical research unit | Phase 3 |
| Lee's Pharmaceutical Limited | Phase 3 |
Medical Subject Heading (MeSH) Categories for LOXAPINE
US Patents and Regulatory Information for LOXAPINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elite Labs Inc | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 076868-001 | Aug 4, 2005 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lannett Co Inc | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 090695-003 | Sep 26, 2011 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Elite Labs Inc | LOXAPINE SUCCINATE | loxapine succinate | CAPSULE;ORAL | 076868-004 | Aug 4, 2005 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for LOXAPINE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Ferrer Internacional S.A. | Adasuve | loxapine | EMEA/H/C/002400 Adasuve is indicated for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. Patients should receive regular treatment immediately after control of acute agitation symptoms. |
Authorised | no | no | no | 2013-02-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


