KOSELUGO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Koselugo, and what generic alternatives are available?
Koselugo is a drug marketed by Astrazeneca and is included in two NDAs. There are eight patents protecting this drug.
This drug has two hundred and one patent family members in forty-five countries.
The generic ingredient in KOSELUGO is selumetinib sulfate. One supplier is listed for this compound. Additional details are available on the selumetinib sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Koselugo
Koselugo was eligible for patent challenges on April 10, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 10, 2028. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for KOSELUGO?
- What are the global sales for KOSELUGO?
- What is Average Wholesale Price for KOSELUGO?
Summary for KOSELUGO
| International Patents: | 201 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 129 |
| Clinical Trials: | 12 |
| Patent Applications: | 4,158 |
| Drug Prices: | Drug price information for KOSELUGO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KOSELUGO |
| What excipients (inactive ingredients) are in KOSELUGO? | KOSELUGO excipients list |
| DailyMed Link: | KOSELUGO at DailyMed |
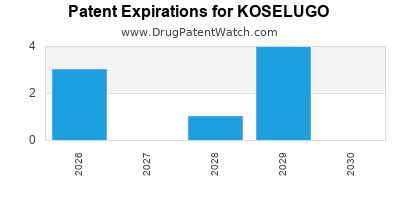

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KOSELUGO
Generic Entry Dates for KOSELUGO*:
Constraining patent/regulatory exclusivity:
NEW PATIENT POPULATION NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for KOSELUGO*:
Constraining patent/regulatory exclusivity:
NEW PRODUCT NDA:
Dosage:
GRANULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for KOSELUGO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Alabama at Birmingham | Phase 2 |
| Congressionally Directed Medical Research Programs | Phase 2 |
| Children's Hospital of Philadelphia | Phase 2 |
Pharmacology for KOSELUGO
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Mitogen-Activated Protein Kinase Kinase 1 Inhibitors Mitogen-Activated Protein Kinase Kinase 2 Inhibitors |
US Patents and Regulatory Information for KOSELUGO
KOSELUGO is protected by eight US patents and five FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KOSELUGO is ⤷ Get Started Free.
This potential generic entry date is based on NEW PATIENT POPULATION.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | KOSELUGO | selumetinib sulfate | GRANULE;ORAL | 219943-002 | Sep 10, 2025 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Astrazeneca | KOSELUGO | selumetinib sulfate | GRANULE;ORAL | 219943-001 | Sep 10, 2025 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Astrazeneca | KOSELUGO | selumetinib sulfate | GRANULE;ORAL | 219943-002 | Sep 10, 2025 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Astrazeneca | KOSELUGO | selumetinib sulfate | GRANULE;ORAL | 219943-002 | Sep 10, 2025 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca | KOSELUGO | selumetinib sulfate | CAPSULE;ORAL | 213756-001 | Apr 10, 2020 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for KOSELUGO
When does loss-of-exclusivity occur for KOSELUGO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8696
Patent: SAL DE SULFATO DE HIDROGENO DEL ACIDO 6-(4-BROMO-2-CLORO-FENILAMINO)-7-FLUOR-3-METIL-3H-BENZOIMIDAZOL-5-CARBOXILICO.
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 06330759
Patent: Novel hydrogen sulfate salt
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0620091
Patent: sal hidrogenossulafato, método para preparo e uso do mesmo
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 34149
Patent: NOUVEAU SEL HYDROGENOSULFATE (NOVEL HYDROGEN SULFATE SALT)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1360718
Patent: Novel hydrogen sulfate salt
Estimated Expiration: ⤷ Get Started Free
Patent: 2329270
Patent: Hydrogen sulfate salt
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0130663
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 14303
Estimated Expiration: ⤷ Get Started Free
Patent: 21030
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 68948
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 088597
Patent: SAL DE SULFATO DE HIDRÓGENO NOVEDOSA
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 68948
Patent: NOUVEAU SEL HYDROGENOSULFATE (NOVEL HYDROGEN SULFATE SALT)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0210043
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1051
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 24043
Patent: NOVEL HYDROGEN SULFATE SALT
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 100046
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2224
Patent: מלח מימן גופרתי של 6-(4-ברומו-2-כלורו-פנילאמינו)-7-פלואורו-3-מתיל-h3-בנזואימידאזול-5-חומצה קרבוקסילית (2-הידרוקסי-אתוקסי)-אמיד לטיפול במחלות המטופלות ע''י עכבה של mek (Hydrogen sulfate salt of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3h-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide for treating diseases treated by inhibition of mek)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 27723
Estimated Expiration: ⤷ Get Started Free
Patent: 09521487
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 968948
Estimated Expiration: ⤷ Get Started Free
Patent: 2021530
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0234
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7733
Patent: NOVEL HYDROGEN SULFATE SALT
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 08008298
Patent: SAL DE SULFATO DE HIDROGENO NOVEDOSA. (NOVEL HYDROGEN SULFATE SALT.)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1139
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9792
Patent: Novel hydrogen sulphate benzimidazole salt
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 68948
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 68948
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 18790
Patent: НОВАЯ ГИДРОСУЛЬФАТНАЯ СОЛЬ (NOVEL HYDROSULPHATE SALT)
Estimated Expiration: ⤷ Get Started Free
Patent: 08129199
Patent: НОВАЯ ГИДРОСУЛЬФАТНАЯ СОЛЬ
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 843
Patent: NOVA SO VODONIK SULFATA (NOVEL HYDROGEN SULFATE SALT)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 68948
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0805705
Patent: NOVEL HYDROGEN SULFATE SALT
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1361460
Estimated Expiration: ⤷ Get Started Free
Patent: 080080200
Patent: NOVEL HYDROGEN SULFATE SALT
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 21746
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 05756
Estimated Expiration: ⤷ Get Started Free
Patent: 0800915
Patent: Novel hydrogen sulfate salt
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 531
Patent: НОВИЙ ГІДРОСУЛЬФАТ[НОВЫЙ ГИДРОСУЛЬФАТ (NOVEL HYDROGEN SULFATE SALT)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KOSELUGO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Ukraine | 101654 | МАТРИКСНАЯ ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ НА ОСНОВЕ ГИДРОСУЛЬФАТА ( 2-ГИДРОКСИЭТОКСИ)АМИДА 6-( 4-БРОМ-2-ХЛОРФЕНИЛАМИНО)-7-ФТОР-3-МЕТИЛ-3Н-БЕНЗОИМИДАЗОЛ-5-КАРБОНОВОЙ КИСЛОТЫ;МАТРИКСНА ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ НА ОСНОВІ ГІДРОСУЛЬФАТУ (2-ГІДРОКСІЕТОКСІ)АМІДУ 6-(4-БРОМ-2-ХЛОРФЕНІЛАМІНО)-7-ФТОР-3-МЕТИЛ-3Н-БЕНЗОІМІДАЗОЛ-5-КАРБОНОВОЇ КИСЛОТИ (MATRIX PHARMACEUTICAL COMPOSITION BASED ON HYDROGEN SULPHATE SALT OF 6-(4-BROMO-2-CHLORO-PHENYLAMINO)-7-FLUORO-3-METHYL-3H-BENZOIMIDAZOLE-5-CARBOXYLIC ACID(2-HYDROXY-ETHOXY)-AMIDE) | ⤷ Get Started Free |
| Peru | 20091755 | COMPOSICION FARMACEUTICA QUE COMPRENDE UNA SAL DE SULFATO DE HIDROGENO DE 6-(4-BROMO-2-CLORO-FENILAMINO)-7-FLUORO-3-METIL-3H-BENZOIMIDAZOL-5-ACIDO CARBOXILICO (2-HIDROXI-ETOXI)-AMIDA | ⤷ Get Started Free |
| Russian Federation | 2491920 | ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ 271 (PHARMACEUTICAL COMPOSITION 271) | ⤷ Get Started Free |
| Lithuania | PA2021530 | ⤷ Get Started Free | |
| Japan | 2008019277 | N3 ALKYLATED BENZIMIDAZOLE AS MEK INHIBITOR | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KOSELUGO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1968948 | C20210036 00434 | Estonia | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIIB;REG NO/DATE: EU/1/21/1552 19.06.2021 |
| 1482932 | 648 | Finland | ⤷ Get Started Free | |
| 1968948 | 301139 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIBWATERSTOFSULFAAT, DESGEWENST IN WATERVRIJE VORM OF IN DE VORM VAN EEN SOLVAAT; REGISTRATION NO/DATE: EU/1/21/1552 20210619 |
| 1968948 | 2190048-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIB HYDROGEN SULFATE, INCLUDING ANY SOLVATES AND ANHYDROUS FORMS THEREOF; REG. NO/DATE: EU/1/21/1552 20210619 |
| 1482932 | C201930018 | Spain | ⤷ Get Started Free | PRODUCT NAME: BINIMETINIB O UNA SAL O UN SOLVATO FARMACEUTICAMENTE ACEPTABLE DEL MISMO.; NATIONAL AUTHORISATION NUMBER: EU/1/18/1315; DATE OF AUTHORISATION: 20180920; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1315; DATE OF FIRST AUTHORISATION IN EEA: 20180920 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for KOSELUGO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
