KERENDIA Drug Patent Profile
✉ Email this page to a colleague
When do Kerendia patents expire, and when can generic versions of Kerendia launch?
Kerendia is a drug marketed by Bayer Hlthcare and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has ninety-six patent family members in forty-nine countries.
The generic ingredient in KERENDIA is finerenone. One supplier is listed for this compound. Additional details are available on the finerenone profile page.
DrugPatentWatch® Generic Entry Outlook for Kerendia
Kerendia was eligible for patent challenges on July 9, 2025.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 12, 2029. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for KERENDIA?
- What are the global sales for KERENDIA?
- What is Average Wholesale Price for KERENDIA?
Summary for KERENDIA
| International Patents: | 96 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 33 |
| Clinical Trials: | 6 |
| Patent Applications: | 325 |
| Drug Prices: | Drug price information for KERENDIA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KERENDIA |
| What excipients (inactive ingredients) are in KERENDIA? | KERENDIA excipients list |
| DailyMed Link: | KERENDIA at DailyMed |
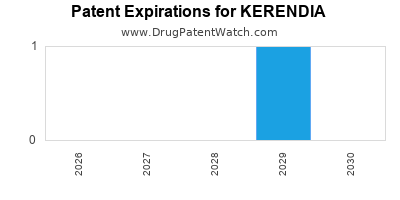
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KERENDIA
Generic Entry Date for KERENDIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for KERENDIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Boehringer Ingelheim | Phase 4 |
| University Medical Center Groningen | Phase 4 |
| University of North Carolina, Chapel Hill | Phase 2 |
Pharmacology for KERENDIA
| Drug Class | Nonsteroidal Mineralocorticoid-Receptor Antagonist |
| Mechanism of Action | Mineralocorticoid Receptor Antagonists |
Paragraph IV (Patent) Challenges for KERENDIA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KERENDIA | Tablets | finerenone | 10 mg and 20 mg | 215341 | 9 | 2025-07-09 |
US Patents and Regulatory Information for KERENDIA
KERENDIA is protected by two US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KERENDIA is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,436,180.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | 8,436,180 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | 8,436,180 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-001 | Jul 9, 2021 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-003 | Jul 11, 2025 | RX | Yes | No | 8,436,180 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bayer Hlthcare | KERENDIA | finerenone | TABLET;ORAL | 215341-002 | Jul 9, 2021 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for KERENDIA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bayer AG | Kerendia | finerenone | EMEA/H/C/005200Kerendia is indicated for the treatment of chronic kidney disease (stage 3 and 4 with albuminuria) associated with type 2 diabetes in adults. | Authorised | no | no | no | 2022-02-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for KERENDIA
When does loss-of-exclusivity occur for KERENDIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5463
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08221071
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0808098
Estimated Expiration: ⤷ Get Started Free
Patent: 2020008544
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 79232
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08000502
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1641352
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 20951
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 976
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150702
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 874
Estimated Expiration: ⤷ Get Started Free
Patent: 090148
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16455
Estimated Expiration: ⤷ Get Started Free
Patent: 22022
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 32206
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 009000205
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 099581
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 32206
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1017
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2007009494
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 0900230
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 09001597
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 40194
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 26441
Estimated Expiration: ⤷ Get Started Free
Patent: 200015
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 0060
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 67586
Estimated Expiration: ⤷ Get Started Free
Patent: 52754
Estimated Expiration: ⤷ Get Started Free
Patent: 10519232
Estimated Expiration: ⤷ Get Started Free
Patent: 14012678
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 18
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 2022512
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0260
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0748
Estimated Expiration: ⤷ Get Started Free
Patent: 6873
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09008701
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 245
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1192
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9230
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 22013
Estimated Expiration: ⤷ Get Started Free
Panama
Patent: 70101
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 090724
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 32206
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 32206
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 70932
Estimated Expiration: ⤷ Get Started Free
Patent: 09135659
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 290071
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 32206
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0905730
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1614164
Estimated Expiration: ⤷ Get Started Free
Patent: 090129992
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 40803
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 15608
Estimated Expiration: ⤷ Get Started Free
Patent: 74821
Estimated Expiration: ⤷ Get Started Free
Patent: 0843755
Estimated Expiration: ⤷ Get Started Free
Patent: 1340968
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 09000318
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2065
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 931
Estimated Expiration: ⤷ Get Started Free
Patent: 952
Estimated Expiration: ⤷ Get Started Free
Patent: 953
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KERENDIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Brazil | PI0808098 | ⤷ Get Started Free | |
| South Korea | 20180041138 | (4S)-4--5-에톡시-2,8-디메틸-1,4-디히드로-1,6-나프티리딘-3-카르복스아미드의 제조 방법 및 활성 제약 성분으로서 사용하기 위한 그의 정제 | ⤷ Get Started Free |
| Hungary | E026441 | ⤷ Get Started Free | |
| Portugal | 2132206 | ⤷ Get Started Free | |
| Mexico | 367960 | PROCEDIMIENTO PARA LA PREPARACION DE (4S)-4-(4-CIANO-2-METOXIFENIL O)-5-ETOXI-2,8-DIMETILO-1,4-DIHIDRO-1,6-NAFTIRIDINA-3-CARBOXAMIDA Y SU PURIFICACION PARA SU USO COMO PRINCIPIO ACTIVO FARMACEUTICO. (METHOD FOR THE PREPARATION OF (4S)-4-(4-CYANO-2-METHOXYPHENYL)-5- ETHOXY-2,8-DIMETHYL-1,4-DIHYDRO-1-6-NAPHTHYRIDINE-3-CARBOXAMIDE AND THE PURIFICATION THEREOF FOR USE AS AN ACTIVE PHARMACEUTICAL INGREDIENT.) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KERENDIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2132206 | 15/2022 | Austria | ⤷ Get Started Free | PRODUCT NAME: FINERENON UND SALZE, SOLVATE UND SOLVATE DER SALZE DAVON; REGISTRATION NO/DATE: EU/1/21/1616 (MITTEILUNG) 20220217 |
| 2132206 | LUC00260 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: FINERENONE, SES SELS ET SOLVATES AINSI QUE LES SOLVATES DES SELS DE FINERENONE; AUTHORISATION NUMBER AND DATE: EU/1/21/1616 20220217 |
| 2132206 | PA2022512,C2132206 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: FINERENONAS; REGISTRATION NO/DATE: EU/1/21/1616 20220216 |
| 2132206 | PA2022512 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: FINERENONAS; REGISTRATION NO/DATE: EU/1/21/1616 20220216 |
| 2132206 | C202230028 | Spain | ⤷ Get Started Free | PRODUCT NAME: FINERENONA Y SUS SALES, SOLVATOS Y SOLVATOS DE LAS SALES; NATIONAL AUTHORISATION NUMBER: EU/1/21/1616; DATE OF AUTHORISATION: 20220216; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/21/1616; DATE OF FIRST AUTHORISATION IN EEA: 20220216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
