INVEGA TRINZA Drug Patent Profile
✉ Email this page to a colleague
When do Invega Trinza patents expire, and what generic alternatives are available?
Invega Trinza is a drug marketed by Janssen Pharms and is included in one NDA. There is one patent protecting this drug and three Paragraph IV challenges.
This drug has fifty-eight patent family members in twenty-seven countries.
The generic ingredient in INVEGA TRINZA is paliperidone palmitate. There are thirty-eight drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the paliperidone palmitate profile page.
DrugPatentWatch® Generic Entry Outlook for Invega Trinza
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are five tentative approvals for the generic drug (paliperidone palmitate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for INVEGA TRINZA?
- What are the global sales for INVEGA TRINZA?
- What is Average Wholesale Price for INVEGA TRINZA?
Summary for INVEGA TRINZA
| International Patents: | 58 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 65 |
| Patent Applications: | 382 |
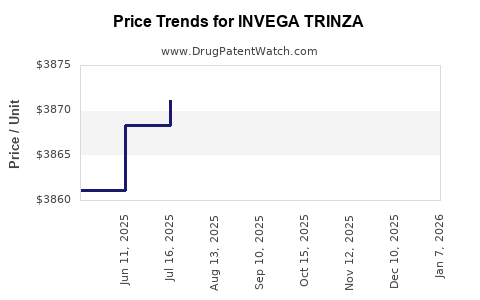
| Drug Prices: | Drug price information for INVEGA TRINZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for INVEGA TRINZA |
| What excipients (inactive ingredients) are in INVEGA TRINZA? | INVEGA TRINZA excipients list |
| DailyMed Link: | INVEGA TRINZA at DailyMed |
Pharmacology for INVEGA TRINZA
| Drug Class | Atypical Antipsychotic |
Paragraph IV (Patent) Challenges for INVEGA TRINZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| INVEGA TRINZA | Extended-release Injectable Suspension | paliperidone palmitate | 273 mg/0.875 mL and 410 mg/1.315 mL | 207946 | 1 | 2021-07-14 |
| INVEGA TRINZA | Extended-release Injectable Suspension | paliperidone palmitate | 819 mg/2.625 mL | 207946 | 1 | 2021-04-30 |
| INVEGA TRINZA | Extended-release Injectable Suspension | paliperidone palmitate | 546 mg/1.75 mL | 207946 | 1 | 2020-06-24 |
US Patents and Regulatory Information for INVEGA TRINZA
INVEGA TRINZA is protected by two US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-001 | May 18, 2015 | RX | Yes | Yes | 10,143,693 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-004 | May 18, 2015 | RX | Yes | Yes | 10,143,693 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-002 | May 18, 2015 | RX | Yes | Yes | 10,143,693 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-003 | May 18, 2015 | RX | Yes | Yes | 10,143,693 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for INVEGA TRINZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-001 | May 18, 2015 | 6,077,843*PED | ⤷ Get Started Free |
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-003 | May 18, 2015 | 6,077,843*PED | ⤷ Get Started Free |
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-002 | May 18, 2015 | 6,077,843*PED | ⤷ Get Started Free |
| Janssen Pharms | INVEGA TRINZA | paliperidone palmitate | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 207946-004 | May 18, 2015 | 6,077,843*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for INVEGA TRINZA
When does loss-of-exclusivity occur for INVEGA TRINZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 16244801
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Patent: 20239611
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Patent: 22221405
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Patent: 24227790
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017021383
Patent: regime de dosagem para doses omitidas de ésteres de paliperidona injetáveis de longa ação
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 25908
Patent: PROGRAMME DE DOSAGE DE DOSES OUBLIEES DESTINE AUX ESTERS DE PALIPERIDONE INJECTABLES A ACTION PROLONGEE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 88401
Patent: PROGRAMME DE DOSAGE DE DOSES OUBLIEES DESTINE AUX ESTERS DE PALIPERIDONE INJECTABLES A ACTION PROLONGEE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0201027
Estimated Expiration: ⤷ Get Started Free
Patent: 0240022
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 23203
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7185
Patent: СПОСОБ ЛЕЧЕНИЯ ПСИХОЗА, ШИЗОФРЕНИИ ИЛИ БИПОЛЯРНОГО РАССТРОЙСТВА (METHOD OF TREATING PSYCHOSIS, SCHIZOPHRENIA OR BIPOLAR DISORDER)
Estimated Expiration: ⤷ Get Started Free
Patent: 1792209
Patent: СХЕМА ВВЕДЕНИЯ ПРОПУЩЕННЫХ ДОЗ ИНЪЕКЦИОННЫХ СЛОЖНЫХ ЭФИРОВ ПАЛИПЕРИДОНА ДЛИТЕЛЬНОГО ДЕЙСТВИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 80416
Patent: SCHÉMA DE TRAITEMENT EN CAS DE DOSES OUBLIÉES POUR DES ESTERS DE PALIPÉRIDONE INJECTABLES À ACTION PROLONGÉE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Patent: SCHÉMA DE TRAITEMENT EN CAS DE DOSES OUBLIÉES POUR DES ESTERS DE PALIPÉRIDONE INJECTABLES À ACTION PROLONGÉE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 49323
Patent: SCHÉMA POSOLOGIQUE POUR DOSES MANQUÉES POUR ESTERS DE PALIPÉRIDONE INJECTABLES À ACTION PROLONGÉE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 49047
Patent: 用於長效可注射的帕利酮酯的遺漏劑量的投藥療程 (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 49485
Estimated Expiration: ⤷ Get Started Free
Patent: 65435
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4669
Patent: משטר מינון עבור מינונים חסרים של פליפרידון אסטרים הניתנים להזרקה עם פעילות ארוכת טווח (Dosing regimen for missed doses for long-acting injectable paliperidone esters)
Estimated Expiration: ⤷ Get Started Free
Patent: 9340
Patent: משטר מינון עבור מינונים חסרים של פליפרידון אסטרים הניתנים להזרקה עם פעילות ארוכת טווח (Dosing regimen for missed doses for long-acting injectable paliperidone esters)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 28221
Estimated Expiration: ⤷ Get Started Free
Patent: 28503
Estimated Expiration: ⤷ Get Started Free
Patent: 22277
Estimated Expiration: ⤷ Get Started Free
Patent: 18510894
Patent: 長時間作用型注射可能パリペリドンエステルの抜かした投与量のための投与レジメン
Estimated Expiration: ⤷ Get Started Free
Patent: 20090498
Patent: 長時間作用型注射可能パリペリドンエステルの抜かした投与量のための投与レジメン (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 21130680
Patent: 長時間作用型注射可能パリペリドンエステルの抜かした投与量のための投与レジメン (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 917
Patent: Schéma de traitement en cas de doses oubliées pour des esters de palipéridone injectables à action prolongée
Estimated Expiration: ⤷ Get Started Free
Patent: 511
Patent: SCHÉMA DE TRAITEMENT EN CAS DE DOSES OUBLIÉES POUR DES ESTERS DE PALIPÉRIDONE INJECTABLES À ACTION PROLONGÉE
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5952
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Patent: 8246
Patent: Dosing regimen for missed doses for long-acting injectable paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 02000351
Estimated Expiration: ⤷ Get Started Free
Patent: 02300478
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 510
Patent: DOZNI REŽIM ZA PROPUŠTENE DOZE INJEKTIBILNIH ESTARA PALIPERIDONA SA PRODUŽENIM DELOVANJEM (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 024
Patent: DOZNI REŽIM ZA PROPUŠTENE DOZE INJEKTIBILNIH ESTARA PALIPERIDONA SA PRODUŽENIM DELOVANJEM (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 80416
Estimated Expiration: ⤷ Get Started Free
Patent: 44326
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2606678
Estimated Expiration: ⤷ Get Started Free
Patent: 2755145
Estimated Expiration: ⤷ Get Started Free
Patent: 170134583
Patent: 장기간 작용형 주사용 팔리페리돈 에스테르에 대한 누락된 용량에 대한 투여 계획
Estimated Expiration: ⤷ Get Started Free
Patent: 230162162
Patent: 장기간 작용형 주사용 팔리페리돈 에스테르에 대한 누락된 용량에 대한 투여 계획 (Dosing regimen for missed doses for long-acting injectable paliperidone esters)
Estimated Expiration: ⤷ Get Started Free
Patent: 250013293
Patent: 장기간 작용형 주사용 팔리페리돈 에스테르에 대한 누락된 용량에 대한 투여 계획 (Dosing regimen for missed doses for long-acting injectable paliperidone esters)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 02299
Estimated Expiration: ⤷ Get Started Free
Patent: 67585
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 94825
Estimated Expiration: ⤷ Get Started Free
Patent: 1642863
Patent: Dosing regimen for missed doses for long-acting injectable Paliperidone esters
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 8732
Patent: СХЕМА ВВЕДЕННЯ ПРОПУЩЕНИХ ДОЗ ЕСТЕРІВ ПАЛІПЕРИДОНУ ТРИВАЛОЇ ДІЇ ДЛЯ ІН'ЄКЦІЙНОГО ЗАСТОСУВАННЯ (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering INVEGA TRINZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 4025187 | ⤷ Get Started Free | |
| Japan | 2023552149 | 持続放出パリペリドン注射可能製剤に関連する投与レジメン | ⤷ Get Started Free |
| European Patent Office | 3744326 | SCHÉMA DE TRAITEMENT EN CAS DE DOSES OUBLIÉES POUR DES ESTERS DE PALIPÉRIDONE INJECTABLES À ACTION PROLONGÉE (DOSING REGIMEN FOR MISSED DOSES FOR LONG-ACTING INJECTABLE PALIPERIDONE ESTERS) | ⤷ Get Started Free |
| South Korea | 20230162162 | 장기간 작용형 주사용 팔리페리돈 에스테르에 대한 누락된 용량에 대한 투여 계획 (Dosing regimen for missed doses for long-acting injectable paliperidone esters) | ⤷ Get Started Free |
| Estonia | 9800136 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for INVEGA TRINZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0904081 | SPC/GB11/044 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: PALIPERIDONE PALMITATE; REGISTERED: UK EU/1/11/672/001 20110304; UK EU/1/11/672/002 20110304; UK EU/1/11/672/003 20110304; UK EU/1/11/672/004 20110304; UK EU/1/11/672/005 20110304; UK EU/1/11/672/006 20110304 |
| 0904081 | 2011/021 | Ireland | ⤷ Get Started Free | PRODUCT NAME: PALIPERIDONE PALMITATE ESTER; REGISTRATION NO/DATE: EU/1/11/672/001-006 20110304 |
| 0904081 | 91842 | Luxembourg | ⤷ Get Started Free | 91842, EXPIRES: 20220512 |
| 0904081 | 1190023-0 | Sweden | ⤷ Get Started Free | PRODUCT NAME: PALIPERIDONPALMITAT; REG. NO/DATE: EU/1/11/672/001-006 20110304 |
| 0368388 | 07C0044 | France | ⤷ Get Started Free | PRODUCT NAME: PALIPERIDONE; REGISTRATION NO/DATE IN FRANCE: EU/1/07/395/01 DU 20070626; REGISTRATION NO/DATE AT EEC: EU/1/07/395/01 DU 20070625 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for INVEGA TRINZA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
