INBRIJA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Inbrija, and when can generic versions of Inbrija launch?
Inbrija is a drug marketed by Merz and is included in one NDA. There are five patents protecting this drug.
This drug has ninety-eight patent family members in twenty-two countries.
The generic ingredient in INBRIJA is levodopa. There are eighteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the levodopa profile page.
DrugPatentWatch® Generic Entry Outlook for Inbrija
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 16, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for INBRIJA?
- What are the global sales for INBRIJA?
- What is Average Wholesale Price for INBRIJA?
Summary for INBRIJA
| International Patents: | 98 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 134 |
| Drug Prices: | Drug price information for INBRIJA |
| What excipients (inactive ingredients) are in INBRIJA? | INBRIJA excipients list |
| DailyMed Link: | INBRIJA at DailyMed |
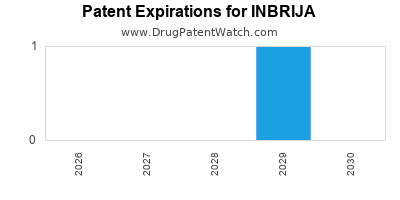

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for INBRIJA
Generic Entry Date for INBRIJA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INHALATION |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for INBRIJA
| Drug Class | Aromatic Amino Acid |
US Patents and Regulatory Information for INBRIJA
INBRIJA is protected by five US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of INBRIJA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for INBRIJA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Merz | INBRIJA | levodopa | POWDER;INHALATION | 209184-001 | Dec 21, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for INBRIJA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Acorda Therapeutics Ireland Limited | Inbrija | levodopa | EMEA/H/C/004786Inbrija is indicated for the intermittent treatment of episodic motor fluctuations (OFF episodes) in adult patients with Parkinson’s disease (PD) treated with a levodopa/dopa-decarboxylase inhibitor. | Authorised | no | no | no | 2019-09-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for INBRIJA
When does loss-of-exclusivity occur for INBRIJA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13342246
Patent: High dose levodopa capsules for pulmonary use
Estimated Expiration: ⤷ Get Started Free
Patent: 13342247
Patent: Dosator for filling a capsule with powder
Estimated Expiration: ⤷ Get Started Free
Patent: 13342248
Patent: Ultra low density pulmonary powders
Estimated Expiration: ⤷ Get Started Free
Patent: 17279626
Patent: Dosator For Filling A Capsule With Powder
Estimated Expiration: ⤷ Get Started Free
Patent: 18204674
Patent: High Dose Levodopa Capsules For Pulmonary Use
Estimated Expiration: ⤷ Get Started Free
Patent: 18222983
Patent: Ultra Low Density Pulmonary Powders
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015010601
Estimated Expiration: ⤷ Get Started Free
Patent: 2015010603
Patent: CÁPSULAS DE LEVODOPA DE DOSE ALTA PARA O USO PULMONAR
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 90451
Patent: CAPSULES DE LEVODOPA A DOSE ELEVEE POUR UNE UTILISATION PULMONAIRE (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 90454
Patent: APPAREIL DE DOSAGE POUR REMPLIR UNE CAPSULE AVEC UNE POUDRE SECHE (DOSATOR FOR FILLING A CAPSULE WITH POWDER)
Estimated Expiration: ⤷ Get Started Free
Patent: 90459
Patent: POUDRES PULMONAIRES DE DENSITE ULTRA BASSE (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4918607
Patent: High dose levodopa capsules for pulmonary use
Estimated Expiration: ⤷ Get Started Free
Patent: 5120843
Patent: Ultra low density pulmonary powders
Estimated Expiration: ⤷ Get Started Free
Patent: 9106697
Patent: 用于肺部使用的高剂量左旋多巴胶囊 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 0833539
Patent: 超低密度的肺部粉末 (Ultra low density pulmonary powders)
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 16821
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 16821
Patent: CAPSULES DE LÉVODOPA À DOSE ÉLEVÉE POUR UNE UTILISATION PULMONAIRE (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 16826
Patent: POUDRES PULMONAIRES DE DENSITÉ ULTRA BASSE (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 25611
Patent: APPAREIL DE DOSAGE POUR REMPLIR UNE CAPSULE AVEC UNE POUDRE SÈCHE (DOSATOR FOR FILLING A CAPSULE WITH POWDER)
Estimated Expiration: ⤷ Get Started Free
Patent: 15679
Patent: CAPSULES DE LÉVODOPA À DOSE ÉLEVÉE POUR UNE UTILISATION PULMONAIRE (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 57301
Patent: POUDRES PULMONAIRES DE DENSITÉ ULTRA BASSE (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 12884
Patent: 超低密度的肺部粉末 (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 13186
Patent: 用於肺部使用的高劑量左旋多巴膠囊 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 13187
Patent: 超低密度的肺部粉末 (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 13535
Patent: 用粉末填充膠囊的劑量分配器 (DOSATOR FOR FILLING A CAPSULE WITH POWDER)
Estimated Expiration: ⤷ Get Started Free
Patent: 14957
Patent: 用於肺部使用的高劑量左旋多巴膠囊 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 47786
Estimated Expiration: ⤷ Get Started Free
Patent: 48501
Estimated Expiration: ⤷ Get Started Free
Patent: 69808
Estimated Expiration: ⤷ Get Started Free
Patent: 21629
Estimated Expiration: ⤷ Get Started Free
Patent: 36834
Estimated Expiration: ⤷ Get Started Free
Patent: 15536197
Patent: 乾燥粉末を含むカプセルを充填する投与装置
Estimated Expiration: ⤷ Get Started Free
Patent: 15536988
Patent: 肺使用のための高服用量のレボドパを含むカプセル
Estimated Expiration: ⤷ Get Started Free
Patent: 15536989
Patent: 超低密度肺粉剤
Estimated Expiration: ⤷ Get Started Free
Patent: 18150355
Patent: 肺使用のための高服用量のレボドパを含むカプセル (CAPSULES CONTAINING HIGH DOSES OF LEVODOPA FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 18162258
Patent: 超低密度肺粉剤 (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19213867
Patent: 乾燥粉末を含むカプセルを充填する投与装置 (DOSING APPARATUS FOR FILLING CAPSULE WITH DRY POWDER)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1008
Patent: POLVOS PULMONARES DE ULTRA BAJA DENSIDAD. (ULTRA LOW DENSITY PULMONARY POWDERS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 7602
Patent: CÁPSULAS CON ALTAS DOSIS DE LEVODOPA PARA USO PULMONAR. (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 2999
Patent: POLVOS PULMONARES DE ULTRA BAJA DENSIDAD (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 15005767
Patent: CÁPSULAS CON ALTAS DOSIS DE LEVODOPA PARA USO PULMONAR. (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 15005768
Patent: POLVOS PULMONARES DE ULTRA BAJA DENSIDAD. (ULTRA LOW DENSITY PULMONARY POWDERS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 20012506
Patent: CAPSULAS CON ALTAS DOSIS DE LEVODOPA PARA USO PULMONAR. (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8682
Patent: Dosator apparatus for filling a capsule with dry powder
Estimated Expiration: ⤷ Get Started Free
Patent: 8684
Patent: High dose levodopa capsules for pulmonary use
Estimated Expiration: ⤷ Get Started Free
Patent: 3459
Patent: Dosator for filling a capsule with dry powder
Estimated Expiration: ⤷ Get Started Free
Patent: 7376
Patent: High dose levodopa capsules for pulmonary use
Estimated Expiration: ⤷ Get Started Free
Patent: 7378
Patent: Ultra low density pulmonary powders
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 16821
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 16821
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 70987
Patent: ПОРОШКИ ДЛЯ ИНГАЛЯЦИИ С УЛЬТРАНИЗКОЙ ПЛОТНОСТЬЮ (ULTRA LOW DENSITY INHALATION POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 76093
Patent: КАПСУЛЫ, СОДЕРЖАЩИЕ ВЫСОКИЕ ДОЗЫ ЛЕВОДОПЫ, ДЛЯ ПРИМЕНЕНИЯ ПУТЕМ ИНГАЛЯЦИИ (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 15121091
Patent: ПОРОШКИ ДЛЯ ИНГАЛЯЦИИ С УЛЬТРАНИЗКОЙ ПЛОТНОСТЬЮ
Estimated Expiration: ⤷ Get Started Free
Patent: 15121092
Patent: КАПСУЛЫ, СОДЕРЖАЩИЕ ВЫСОКИЕ ДОЗЫ ЛЕВОДОПЫ, ДЛЯ ПРИМЕНЕНИЯ ПУТЕМ ИНГАЛЯЦИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 18144622
Patent: КАПСУЛЫ, СОДЕРЖАЩИЕ ВЫСОКИЕ ДОЗЫ ЛЕВОДОПЫ, ДЛЯ ПРИМЕНЕНИЯ ПУТЕМ ИНГАЛЯЦИИ
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201706465X
Patent: HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE
Estimated Expiration: ⤷ Get Started Free
Patent: 201707103S
Patent: ULTRA LOW DENSITY PULMONARY POWDERS
Estimated Expiration: ⤷ Get Started Free
Patent: 202109328Q
Patent: HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE
Estimated Expiration: ⤷ Get Started Free
Patent: 201503543P
Patent: HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE
Estimated Expiration: ⤷ Get Started Free
Patent: 201503547T
Patent: ULTRA LOW DENSITY PULMONARY POWDERS
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1504058
Patent: HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE
Estimated Expiration: ⤷ Get Started Free
Patent: 1504060
Patent: ULTRA LOW DENSITY PULMONARY POWDERS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2257164
Estimated Expiration: ⤷ Get Started Free
Patent: 2337781
Estimated Expiration: ⤷ Get Started Free
Patent: 2389785
Estimated Expiration: ⤷ Get Started Free
Patent: 2735396
Estimated Expiration: ⤷ Get Started Free
Patent: 150108816
Patent: ULTRA LOW DENSITY PULMONARY POWDERS
Estimated Expiration: ⤷ Get Started Free
Patent: 150110480
Patent: 폐에서의 사용을 위한 고용량 레보도파 캡슐 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 210062730
Patent: 폐에서의 사용을 위한 고용량 레보도파 캡슐 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 210152020
Patent: 폐용 초저밀도 분말 (ULTRA LOW DENSITY PULMONARY POWDERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 220054703
Patent: 폐에서의 사용을 위한 고용량 레보도파 캡슐 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 230116102
Patent: 폐에서의 사용을 위한 고용량 레보도파 캡슐 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 240134230
Patent: 폐에서의 사용을 위한 고용량 레보도파 캡슐 (HIGH DOSE LEVODOPA CAPSULES FOR PULMONARY USE)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 44153
Estimated Expiration: ⤷ Get Started Free
Patent: 80271
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering INBRIJA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 03043585 | ⤷ Get Started Free | |
| South Korea | 102735396 | ⤷ Get Started Free | |
| Denmark | 1296663 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for INBRIJA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3209302 | CR 2023 00015 | Denmark | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION AF FOSLEVODOPA ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF OG FOSCARBIDOPA ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; NAT. REG. NO/DATE: 66549 (DK) 20221205; FIRST REG. NO/DATE: AT 141371 20220826 |
| 3209302 | C202330028 | Spain | ⤷ Get Started Free | PRODUCT NAME: COMBINACION DE FOSLEVODOPA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DE LA MISMA Y FOSCARBIDOPA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DE LA MISM.; NATIONAL AUTHORISATION NUMBER: 88677-SE/H/0415/003/DC; DATE OF AUTHORISATION: 20230220; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): 141371; DATE OF FIRST AUTHORISATION IN EEA: 20220825 |
| 3209302 | 301224 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE VAN FOSLEVODOPA EN FOSCARBIDOPA, ELK DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; NATIONAL REGISTRATION NO/DATE: RVG128752 20221107; FIRST REGISTRATION: AT 141371 20220826 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Inbrija (Levodopa Inhalation Powder)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
