GILENYA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Gilenya, and what generic alternatives are available?
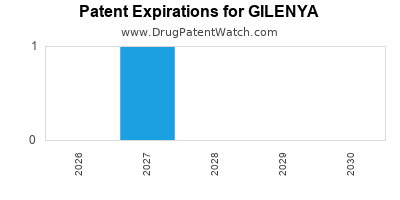
Gilenya is a drug marketed by Novartis and is included in one NDA. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has ninety-eight patent family members in thirty-nine countries.
The generic ingredient in GILENYA is fingolimod hydrochloride. There are twenty-one drug master file entries for this compound. Eighteen suppliers are listed for this compound. Additional details are available on the fingolimod hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Gilenya
A generic version of GILENYA was approved as fingolimod hydrochloride by BIOCON LTD on December 4th, 2019.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for GILENYA?
- What are the global sales for GILENYA?
- What is Average Wholesale Price for GILENYA?
Summary for GILENYA
| International Patents: | 98 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 99 |
| Clinical Trials: | 33 |
| Patent Applications: | 987 |
| Drug Prices: | Drug price information for GILENYA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for GILENYA |
| What excipients (inactive ingredients) are in GILENYA? | GILENYA excipients list |
| DailyMed Link: | GILENYA at DailyMed |

Recent Clinical Trials for GILENYA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Medical University of South Carolina | PHASE2 |
| The Methodist Hospital Research Institute | Phase 2 |
| Genuine Research Center, Egypt | Phase 1 |
Pharmacology for GILENYA
| Drug Class | Sphingosine 1-phosphate Receptor Modulator |
| Mechanism of Action | Sphingosine 1-Phosphate Receptor Modulators |
Paragraph IV (Patent) Challenges for GILENYA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| GILENYA | Capsules | fingolimod hydrochloride | 0.25 mg | 022527 | 1 | 2018-07-19 |
| GILENYA | Capsules | fingolimod hydrochloride | 0.5 mg | 022527 | 19 | 2014-09-22 |
US Patents and Regulatory Information for GILENYA
GILENYA is protected by two US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | GILENYA | fingolimod hydrochloride | CAPSULE;ORAL | 022527-002 | May 11, 2018 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | GILENYA | fingolimod hydrochloride | CAPSULE;ORAL | 022527-001 | Sep 21, 2010 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GILENYA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | GILENYA | fingolimod hydrochloride | CAPSULE;ORAL | 022527-002 | May 11, 2018 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | GILENYA | fingolimod hydrochloride | CAPSULE;ORAL | 022527-001 | Sep 21, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | GILENYA | fingolimod hydrochloride | CAPSULE;ORAL | 022527-001 | Sep 21, 2010 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for GILENYA
When does loss-of-exclusivity occur for GILENYA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5749
Patent: FORMULACIONES
Estimated Expiration: ⤷ Get Started Free
Patent: 4661
Patent: FORMULACIONES
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12236357
Patent: Formulations comprising 2 -amino- 2- [2- (4 - octylphenyl) ethyl] propane -1, 3 - diol
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013024430
Patent: formulações compreendendo 2-amino-2-[2-(4-octilfenil)etil]propano-1,3-diol
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 31600
Patent: FORMULATIONS COMPRENANT 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 13002810
Patent: Composicion farmaceutica solida oral que comprende a) un compuesto 2-amino-2-[2-(4-octil-fenil)-etil]-propano-1,3-diol (fingolimod) en una cantidad de 0,5 mg o menos, o una sal del mismo, b) un relleno y c) un estabilizante que comprende una ciclodextrina; y su uso para tratar una enfermedad autoinmune tal como esclerosis multiple.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3476400
Patent: Formulations comprising 2-amino-2-[2-(4 - octylphenyl) ethyl] propane -1, 3-diol
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 71459
Patent: Formulaciones que comprenden 2-amino-2-[2- (4-octil-fenil)-etil] -propano-1,3-diol
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0170021
Estimated Expiration: ⤷ Get Started Free
Patent: 0200249
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18423
Estimated Expiration: ⤷ Get Started Free
Patent: 22868
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 94037
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 13012912
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-[2-(4-OCTIL-FENIL)-ETIL]-PROPANO-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7721
Patent: ПРЕПАРАТЫ, СОДЕРЖАЩИЕ 2-АМИНО-2-[2-(4-ОКТИЛФЕНИЛ)ЭТИЛ]ПРОПАН-1,3-ДИОЛ (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Patent: 5686
Patent: ПРЕПАРАТЫ, СОДЕРЖАЩИЕ 2-АМИНО-2-[2-(4-ОКТИЛФЕНИЛ)ЭТИЛ]ПРОПАН-1,3-ДИОЛ (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Patent: 1391442
Patent: ПРЕПАРАТЫ, СОДЕРЖАЩИЕ 2-АМИНО-2-[2-(4-ОКТИЛФЕНИЛ)ЭТИЛ]ПРОПАН-1,3-ДИОЛ
Estimated Expiration: ⤷ Get Started Free
Patent: 1790436
Patent: ПРЕПАРАТЫ, СОДЕРЖАЩИЕ 2-АМИНО-2-[2-(4-ОКТИЛФЕНИЛ)ЭТИЛ]ПРОПАН-1,3-ДИОЛ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 94037
Patent: FORMULATIONS COMPRENANT 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Patent: FORMULATIONS COMPRENANT 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1300227
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-(2-(4-OCTIL-FENIL)-ETIL)-PROPANO-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 90309
Patent: 包含 -氨基- -辛基苯基 乙基 丙- -二醇的製劑 (FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL]PROPANE - 1, 3 - DIOL 2--2-[2-(4-)]-13-)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 31286
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8250
Patent: תכשירים המכילים 2-אמינו-2-[2-(4-אוקטילפניל)אתיל]פרופאנ-1,3-דיול (Formulations comprising 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19101
Estimated Expiration: ⤷ Get Started Free
Patent: 14509652
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 77
Patent: تركيبات تتالف من 2-أمينو-2- [ 2- ( 4- أكتيل فينيل ) إثيل ] بروبان - 3, 1- ديول (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 94037
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3746
Patent: FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Patent: 5633
Patent: FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 2522
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-[2- (4-OCTIL-FENIL) -ETIL] -PROPANO-1, 3-DIOL. (FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13011415
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-[2- (4-OCTIL-FENIL) -ETIL] -PROPANO-1, 3-DIOL. (FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 981
Patent: FORMULATIONS COMPRENANT 2-AMINO-2-2-4-OCTYLPHENYL)ETHYL)PROPANE-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5023
Patent: Formulations comprising 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 140162
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-[2-(4-OCTIL-FENIL)-ETIL]-PROPANO-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Patent: 170913
Patent: FORMULACIONES QUE COMPRENDEN 2-AMINO-2-[2-(4-OCTIL-FENIL)-ETIL]-PROPANO-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 94037
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 94037
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 917
Patent: FORMULACIJE KOJE SADRŽE 2-AMINO-2-[2-(4-OKTILFENIL)ETIL]PROPAN-1,3-DIOL (FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3256
Patent: FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 94037
Estimated Expiration: ⤷ Get Started Free
Patent: 43990
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1306636
Patent: FORMULAIONS COMPRISING 2 -AMINO- 2-[2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2027014
Estimated Expiration: ⤷ Get Started Free
Patent: 140014194
Patent: FORMULATIONS COMPRISING 2-AMINO-2-[2-(4-OCTYLPHENYL)ETHYL]PROPANE-1,3-DIOL
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 10966
Estimated Expiration: ⤷ Get Started Free
Patent: 73482
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 28958
Estimated Expiration: ⤷ Get Started Free
Patent: 1244711
Patent: Formulations
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 13000396
Patent: FORMULATIONS COMPRISING 2 -AMINO- 2- [2- (4 - OCTYLPHENYL) ETHYL] PROPANE -1, 3 - DIOL
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 2857
Patent: ПРЕПАРАТ, ЩО МІСТИТЬ 2-АМІНО-2-[2-(4-ОКТИЛФЕНІЛ)ЕТИЛ]ПРОПАН-1,3-ДІОЛ
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering GILENYA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Peru | 20131352 | COMPOSICION FARMACEUTICA QUE CONTIENE UN AGONISTA DEL RECEPTOR S1P Y UN ALCOHOL DE AZUCAR | ⤷ Get Started Free |
| Japan | 2016155840 | ⤷ Get Started Free | |
| Russian Federation | 2012148593 | ПРЕДНАЗНАЧЕННАЯ ДЛЯ ПЕРОРАЛЬНОГО ПРИМЕНЕНИЯ ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ | ⤷ Get Started Free |
| Chile | 2013002810 | Composicion farmaceutica solida oral que comprende a) un compuesto 2-amino-2-[2-(4-octil-fenil)-etil]-propano-1,3-diol (fingolimod) en una cantidad de 0,5 mg o menos, o una sal del mismo, b) un relleno y c) un estabilizante que comprende una ciclodextrina; y su uso para tratar una enfermedad autoinmune tal como esclerosis multiple. | ⤷ Get Started Free |
| Japan | 2013505983 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GILENYA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1613288 | 2011/027 | Ireland | ⤷ Get Started Free | PRODUCT NAME: GILENYA-FINGOLIMOD; NAT REGISTRATION NO/DATE: EU/1/11/677/001 20110317; FIRST REGISTRATION NO/DATE: EU/1/11/677/002 17/03/2011 IRELAND EU/1/11/677/003 17/03/2011 IRELAND EU/1/11/677/004 20110317 |
| 1613288 | CR 2011 00023 | Denmark | ⤷ Get Started Free | PRODUCT NAME: FINGOLIMOD, HERUNDER FINGOLIMOD SOM HYDROCHLORID; REG. NO/DATE: EU/1/11/677/001-004 20110317 |
| 1613288 | 399 | Finland | ⤷ Get Started Free | |
| 1613288 | C01613288/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: FINGOLOMOD; REGISTRATION NO/DATE: SWISSMEDIC 60916 20110103 |
| 1613288 | C300497 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: FINGOLIMOD ALSMEDE FARMACEUTISCH AANVAARDBARE AFGELEIDEN DAARVAN, IN HET BIJZONDER FINGOLIMOD HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/11/677/001-004 20110317 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for GILENYA (Fingolimod): An In-Depth Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
