DOVATO Drug Patent Profile
✉ Email this page to a colleague
When do Dovato patents expire, and when can generic versions of Dovato launch?
Dovato is a drug marketed by Viiv Hlthcare and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and one patent family members in forty-nine countries.
The generic ingredient in DOVATO is dolutegravir sodium; lamivudine. There are seventeen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the dolutegravir sodium; lamivudine profile page.
DrugPatentWatch® Generic Entry Outlook for Dovato
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 24, 2031. This may change due to patent challenges or generic licensing.
There have been twenty-four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for DOVATO?
- What are the global sales for DOVATO?
- What is Average Wholesale Price for DOVATO?
Summary for DOVATO
| International Patents: | 301 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 45 |
| Clinical Trials: | 9 |
| Patent Applications: | 263 |
| Drug Prices: | Drug price information for DOVATO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for DOVATO |
| What excipients (inactive ingredients) are in DOVATO? | DOVATO excipients list |
| DailyMed Link: | DOVATO at DailyMed |
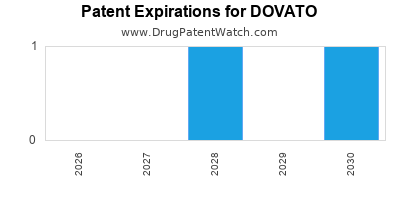
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DOVATO
Generic Entry Date for DOVATO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DOVATO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| ViiV Healthcare | PHASE3 |
| Johns Hopkins University | PHASE3 |
| Saskatchewan Health Authority - Regina Area | PHASE4 |
Pharmacology for DOVATO
Paragraph IV (Patent) Challenges for DOVATO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| DOVATO | Tablets | dolutegravir sodium; lamivudine | 50 mg/300 mg | 211994 | 1 | 2019-07-30 |
US Patents and Regulatory Information for DOVATO
DOVATO is protected by three US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of DOVATO is ⤷ Get Started Free.
This potential generic entry date is based on patent 11,234,985.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | DOVATO | dolutegravir sodium; lamivudine | TABLET;ORAL | 211994-001 | Apr 8, 2019 | RX | Yes | Yes | 11,234,985 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Viiv Hlthcare | DOVATO | dolutegravir sodium; lamivudine | TABLET;ORAL | 211994-001 | Apr 8, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Viiv Hlthcare | DOVATO | dolutegravir sodium; lamivudine | TABLET;ORAL | 211994-001 | Apr 8, 2019 | RX | Yes | Yes | 8,129,385*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for DOVATO
When does loss-of-exclusivity occur for DOVATO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 51
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11209788
Estimated Expiration: ⤷ Get Started Free
Patent: 14202404
Estimated Expiration: ⤷ Get Started Free
Patent: 14202405
Estimated Expiration: ⤷ Get Started Free
Patent: 14202406
Estimated Expiration: ⤷ Get Started Free
Patent: 16204987
Estimated Expiration: ⤷ Get Started Free
Patent: 17268621
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012018670
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 87691
Estimated Expiration: ⤷ Get Started Free
Patent: 67453
Estimated Expiration: ⤷ Get Started Free
Patent: 03988
Estimated Expiration: ⤷ Get Started Free
Patent: 60290
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12002080
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2791129
Estimated Expiration: ⤷ Get Started Free
Patent: 5311033
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 02152
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120423
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150770
Estimated Expiration: ⤷ Get Started Free
Patent: 0180855
Estimated Expiration: ⤷ Get Started Free
Patent: 0181531
Estimated Expiration: ⤷ Get Started Free
Patent: 0240168
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16509
Estimated Expiration: ⤷ Get Started Free
Patent: 20457
Estimated Expiration: ⤷ Get Started Free
Patent: 21040
Estimated Expiration: ⤷ Get Started Free
Patent: 26771
Estimated Expiration: ⤷ Get Started Free
Patent: 18029
Estimated Expiration: ⤷ Get Started Free
Patent: 24017
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 31027
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 012000205
Estimated Expiration: ⤷ Get Started Free
Patent: 021000147
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 12012106
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5176
Estimated Expiration: ⤷ Get Started Free
Patent: 2868
Estimated Expiration: ⤷ Get Started Free
Patent: 7601
Estimated Expiration: ⤷ Get Started Free
Patent: 1290583
Estimated Expiration: ⤷ Get Started Free
Patent: 1690872
Estimated Expiration: ⤷ Get Started Free
Patent: 1892277
Estimated Expiration: ⤷ Get Started Free
Patent: 2190473
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 31027
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 51249
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
Patent: 16599
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0240016
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1043
Estimated Expiration: ⤷ Get Started Free
Patent: C1024
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 79522
Estimated Expiration: ⤷ Get Started Free
Patent: 09629
Estimated Expiration: ⤷ Get Started Free
Patent: 50335
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 26849
Estimated Expiration: ⤷ Get Started Free
Patent: 37812
Estimated Expiration: ⤷ Get Started Free
Patent: 40554
Estimated Expiration: ⤷ Get Started Free
Patent: 65569
Estimated Expiration: ⤷ Get Started Free
Patent: 800042
Estimated Expiration: ⤷ Get Started Free
Patent: 400017
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1007
Estimated Expiration: ⤷ Get Started Free
Patent: 5182
Estimated Expiration: ⤷ Get Started Free
Patent: 7267
Estimated Expiration: ⤷ Get Started Free
Patent: 7658
Estimated Expiration: ⤷ Get Started Free
Patent: 1959
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 68386
Estimated Expiration: ⤷ Get Started Free
Patent: 24619
Estimated Expiration: ⤷ Get Started Free
Patent: 13518107
Estimated Expiration: ⤷ Get Started Free
Patent: 16145204
Estimated Expiration: ⤷ Get Started Free
Patent: 17008087
Estimated Expiration: ⤷ Get Started Free
Patent: 18127473
Estimated Expiration: ⤷ Get Started Free
Patent: 19167371
Estimated Expiration: ⤷ Get Started Free
Patent: 21091705
Estimated Expiration: ⤷ Get Started Free
Patent: 22071126
Estimated Expiration: ⤷ Get Started Free
Patent: 23085431
Estimated Expiration: ⤷ Get Started Free
Patent: 25131664
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 932970
Estimated Expiration: ⤷ Get Started Free
Patent: 494972
Estimated Expiration: ⤷ Get Started Free
Patent: 2018013
Estimated Expiration: ⤷ Get Started Free
Patent: 2024516
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0090
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8334
Estimated Expiration: ⤷ Get Started Free
Patent: 2778
Estimated Expiration: ⤷ Get Started Free
Patent: 7233
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6891
Estimated Expiration: ⤷ Get Started Free
Patent: 7937
Estimated Expiration: ⤷ Get Started Free
Patent: 7938
Estimated Expiration: ⤷ Get Started Free
Patent: 12008774
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 182
Estimated Expiration: ⤷ Get Started Free
Patent: 058
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 002
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1319
Estimated Expiration: ⤷ Get Started Free
Patent: 7824
Estimated Expiration: ⤷ Get Started Free
Patent: 7826
Estimated Expiration: ⤷ Get Started Free
Patent: 7827
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 18036
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 121524
Estimated Expiration: ⤷ Get Started Free
Patent: 160180
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012501537
Estimated Expiration: ⤷ Get Started Free
Patent: 016500195
Estimated Expiration: ⤷ Get Started Free
Patent: 018502489
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 31027
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 31027
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500177
Estimated Expiration: ⤷ Get Started Free
Patent: 01800290
Estimated Expiration: ⤷ Get Started Free
Patent: 01800594
Estimated Expiration: ⤷ Get Started Free
Patent: 02400063
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 123
Estimated Expiration: ⤷ Get Started Free
Patent: 323
Estimated Expiration: ⤷ Get Started Free
Patent: 728
Estimated Expiration: ⤷ Get Started Free
Patent: 183
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 2614
Estimated Expiration: ⤷ Get Started Free
Patent: 201509476R
Estimated Expiration: ⤷ Get Started Free
Patent: 201707183T
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 31027
Estimated Expiration: ⤷ Get Started Free
Patent: 32970
Estimated Expiration: ⤷ Get Started Free
Patent: 27542
Estimated Expiration: ⤷ Get Started Free
Patent: 94972
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1205586
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1830715
Estimated Expiration: ⤷ Get Started Free
Patent: 1883750
Estimated Expiration: ⤷ Get Started Free
Patent: 1964923
Estimated Expiration: ⤷ Get Started Free
Patent: 120128640
Estimated Expiration: ⤷ Get Started Free
Patent: 160111536
Estimated Expiration: ⤷ Get Started Free
Patent: 170078868
Estimated Expiration: ⤷ Get Started Free
Patent: 180078358
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 43066
Estimated Expiration: ⤷ Get Started Free
Patent: 70811
Estimated Expiration: ⤷ Get Started Free
Patent: 88925
Estimated Expiration: ⤷ Get Started Free
Patent: 69969
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 12000376
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1807704
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 5556
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering DOVATO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2531027 | ⤷ Get Started Free | |
| Mexico | 383683 | SINTESIS DE INHIBIDORES DE INTEGRASA DE VIH DE CARBAMOIL-PIRIDONA E INTERMEDIARIOS. (SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES) | ⤷ Get Started Free |
| Singapore | 171308 | SYNTHESIS OF CARBAMOYLPYRIDONE HIV INTEGRASE INHIBITORS AND INTERMEDIATES | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DOVATO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2932970 | 122018000125 | Germany | ⤷ Get Started Free | PRODUCT NAME: EINE KOMBINATION UMFASSEND DOLUTEGRAVIR ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON (Z. B DOLUTEGRAVIR NATRIUM) UND RILPIVIRIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON (Z.B RILPIVIRIN HYDROCHLORID); REGISTRATION NO/DATE: EU/1/18/1282 20180516 |
| 2932970 | 300957 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: EEN COMBINATIE OMVATTENDE DOLUTEGRAVIR OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN (ZOALS DOLUTEGRAVIR NATRIUM) EN RILPIVIRINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN (ZOALS RILPIVIRINE HYDROCHLORIDE); REGISTRATION NO/DATE: EU/1/18/1282 20180518 |
| 2465580 | 21C1023 | France | ⤷ Get Started Free | PRODUCT NAME: CABOTEGRAVIR OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; NAT. REGISTRATION NO/DATE: EU/1/20/1481 20201221; FIRST REGISTRATION: - EU/1/20/1481 20201221 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for DOVATO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
