DAURISMO Drug Patent Profile
✉ Email this page to a colleague
When do Daurismo patents expire, and what generic alternatives are available?
Daurismo is a drug marketed by Pfizer and is included in one NDA. There are five patents protecting this drug.
This drug has ninety-eight patent family members in fifty countries.
The generic ingredient in DAURISMO is glasdegib maleate. One supplier is listed for this compound. Additional details are available on the glasdegib maleate profile page.
DrugPatentWatch® Generic Entry Outlook for Daurismo
Daurismo was eligible for patent challenges on November 21, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 13, 2036. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for DAURISMO?
- What are the global sales for DAURISMO?
- What is Average Wholesale Price for DAURISMO?
Summary for DAURISMO
| International Patents: | 98 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 21 |
| Clinical Trials: | 2 |
| Patent Applications: | 275 |
| Drug Prices: | Drug price information for DAURISMO |
| What excipients (inactive ingredients) are in DAURISMO? | DAURISMO excipients list |
| DailyMed Link: | DAURISMO at DailyMed |
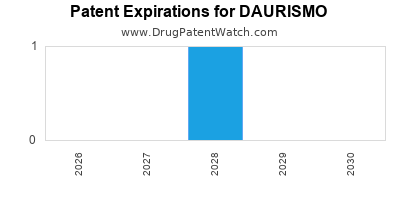

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DAURISMO
Generic Entry Date for DAURISMO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DAURISMO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| City of Hope Medical Center | Phase 1 |
| National Cancer Institute (NCI) | Phase 1 |
| M.D. Anderson Cancer Center | Phase 1/Phase 2 |
Pharmacology for DAURISMO
| Drug Class | Hedgehog Pathway Inhibitor |
| Mechanism of Action | Smoothened Receptor Antagonists |
US Patents and Regulatory Information for DAURISMO
DAURISMO is protected by five US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of DAURISMO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for DAURISMO
When does loss-of-exclusivity occur for DAURISMO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4391
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 16251940
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017021075
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 27736
Estimated Expiration: ⤷ Get Started Free
Patent: 83387
Estimated Expiration: ⤷ Get Started Free
China
Patent: 7531667
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 24056
Estimated Expiration: ⤷ Get Started Free
Patent: 25333
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 86176
Estimated Expiration: ⤷ Get Started Free
Patent: 66768
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 86176
Estimated Expiration: ⤷ Get Started Free
Patent: 66768
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 43416
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 48664
Estimated Expiration: ⤷ Get Started Free
Patent: 59506
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5224
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 45728
Estimated Expiration: ⤷ Get Started Free
Patent: 16204373
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 5384
Estimated Expiration: ⤷ Get Started Free
Patent: 17013645
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5719
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 86176
Estimated Expiration: ⤷ Get Started Free
Patent: 66768
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 86176
Estimated Expiration: ⤷ Get Started Free
Patent: 66768
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 17564
Estimated Expiration: ⤷ Get Started Free
Patent: 17137269
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201707863Q
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 86176
Estimated Expiration: ⤷ Get Started Free
Patent: 66768
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1706391
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2078444
Estimated Expiration: ⤷ Get Started Free
Patent: 170129245
Estimated Expiration: ⤷ Get Started Free
Patent: 190038677
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 74053
Estimated Expiration: ⤷ Get Started Free
Patent: 23593
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 46093
Estimated Expiration: ⤷ Get Started Free
Patent: 1702238
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering DAURISMO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Argentina | 067346 | ⤷ Get Started Free | |
| Taiwan | I646093 | ⤷ Get Started Free | |
| Portugal | 3286176 | ⤷ Get Started Free | |
| European Patent Office | 2170860 | ⤷ Get Started Free | |
| Denmark | 3666768 | ⤷ Get Started Free | |
| Mexico | 375384 | FORMAS CRISTALINAS DE 1-((2R,4R)-2-(1H-BENZO[D]IMIDAZOL-2-IL)-1-METILPIPERIDIN-4-IL)-3-(4-CIANOFENIL)UREA MALEATO. (CRYSTALLINE FORMS OF 1-((<i>2R,4R</i>)-2-(1<i>H</i>-BENZO[D]IMIDAZOL-2-YL)-1-METHYLPIPERIDIN-4-YL)-3-(4-CYANOPHENYL)UREA MALEATE) | ⤷ Get Started Free |
| South Korea | 20190038677 | 1-((2R,4R)-2-(1H-벤조[D]이미다졸-2-일)-1-메틸피페리딘-4-일)-3-우레아 말레에이트의 결정질 형태 (1-2R4R-2-1H-[D]-2--1--4--3-4- CRYSTALLINE FORMS OF 1-2R4R-2-1H-BENZO[D]IMIDAZOL-2-YL-1-METHYLPIPERIDIN-4-YL-3-4-CYANOPHENYLUREA MALEATE) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DAURISMO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2170860 | PA2020528 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIBAS, PASIRINKTINAI FARMACINIU POZIURIU PRIIMTINOS DRUSKOS, ISKAITANT MALEATO DRUSKA, PAVIDALU; REGISTRATION NO/DATE: EU/1/20/1451 20200626 |
| 2170860 | 2090036-1 | Sweden | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE MALEATE SALT; REG. NO/DATE: EU/1/20/1451 20200629 |
| 2170860 | 301057 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, MET INBEGRIP VAN HET MALEAATZOUT; REGISTRATION NO/DATE: EU/1/20/1451/001-004 20200626 |
| 2170860 | 779 | Finland | ⤷ Get Started Free | |
| 2170860 | C20200032 00354 | Estonia | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIIB;REG NO/DATE: EU/1/20/1451 29.06.2020 |
| 2170860 | 20C1038 | France | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIB, OPTIONELLEMENT SOUS LA FORME D'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE, NOTAMMENT LE SEL DE MALEATE; REGISTRATION NO/DATE: EU/1/20/1451 20200629 |
| 2170860 | 2020/034 | Ireland | ⤷ Get Started Free | PRODUCT NAME: GLASDEGIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE MALEATE SALT; REGISTRATION NO/DATE: EU/1/20/1451/001 EU/1/20/1451/004 20200626 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Daurismo
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
