Last updated: August 19, 2025
Introduction
Cefdinir, a third-generation orally administered cephalosporin antibiotic, has become a prominent asset within the antimicrobial pharmacopeia. Its broad-spectrum activity against a variety of bacterial pathogens, along with its favorable dosing profile, has cemented its role in treating respiratory tract infections, otitis media, and skin infections. This analysis examines the current market dynamics and future financial trajectory of Cefdinir, considering competitive landscape shifts, regulatory factors, epidemiological trends, and evolving prescribing behaviors.
Pharmacological Profile and Clinical Positioning
Cefdinir, marketed under brand names such as Omnicef and generic formulations, exhibits potent activity against Gram-positive bacteria, including Streptococcus pneumoniae, and select Gram-negative pathogens like Haemophilus influenzae. Its once-daily oral dosing enhances patient compliance, contributing to its widespread use in outpatient settings. The drug's safety profile is well-established, supporting broad prescribing across pediatric and adult populations.
The clinical adoption of Cefdinir is largely driven by the increasing prevalence of upper respiratory infections and the rising resistance to older agents like amoxicillin. Its versatility in empirical therapy has fostered consistent demand, although its utilization is influenced by clinical guidelines and antimicrobial stewardship initiatives.
Market Size and Segmentation
The global antibiotics market is projected to surpass USD 53 billion annually by 2027, with cephalosporins accounting for a significant share—estimated at approximately 18% [1]. Cefdinir's segment primarily comprises pediatric prescriptions, with the United States being the dominant market due to high childhood infection rates and established prescribing protocols. Emerging markets in Asia-Pacific and Latin America are experiencing rapid growth, driven by expanding healthcare infrastructure and antibiotic availability.
Segment-specific demand is influenced by factors such as:
- Age demographics: Pediatric populations substantially contribute to Cefdinir's sales.
- Infection prevalence: Seasonal spikes in respiratory infections affect sales cycles.
- Prescribing practices: Preference shifts toward topical and targeted therapies impact oral cephalosporin usage.
Competitive Landscape
Cefdinir faces competition from both branded and generic antibiotics, including amoxicillin-clavulanate, azithromycin, and other third-generation cephalosporins such as cefdinir's close cousin, cefixime. The generic manufacturing sector’s expansion has intensified price competition, exerting downward pressure on margins.
Notably, emerging oral antibiotics such as lefamulin and omadacycline are entering the market with potential to challenge traditional agents like Cefdinir in specific indications. Additionally, the increasing adoption of rapid diagnostic tools influences prescriber preferences towards narrow-spectrum agents, potentially impacting Cefdinir's market share.
Regulatory and Safety Considerations
Regulatory bodies like the U.S. Food and Drug Administration (FDA) require ongoing safety monitoring, especially concerning adverse effects like diarrhea, hypersensitivity, and potential for Clostridioides difficile infections. Recent updates emphasizing antimicrobial stewardship aim to mitigate overprescription, affecting Cefdinir's demand.
Furthermore, resistance development warrants close surveillance. While Cefdinir remains effective against many pathogens, increasing resistance among certain bacterial strains necessitates cautious use and influences prescribing guidelines.
Epidemiological Trends and Impact
The rising incidence of respiratory infections—both viral and bacterial—in pediatric and adult populations sustains Cefdinir demand. However, viral etiology predominates many respiratory illnesses, leading to potential misuse of antibiotics. The awareness campaigns and guidelines emphasizing judicious antibiotic use are gradually shaping prescribing behavior, which could modulate Cefdinir's market growth.
The COVID-19 pandemic further impacted demand dynamics, with a temporary decline in outpatient prescriptions during lockdowns, followed by a resurgence driven by unmet bacterial infection needs post-pandemic.
Financial Trajectory
Historical Performance
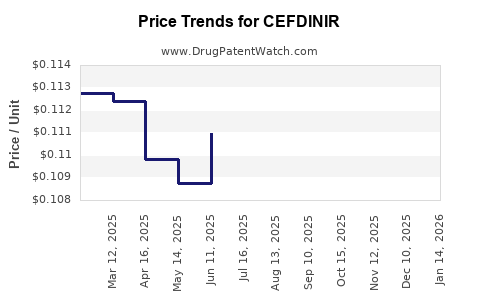
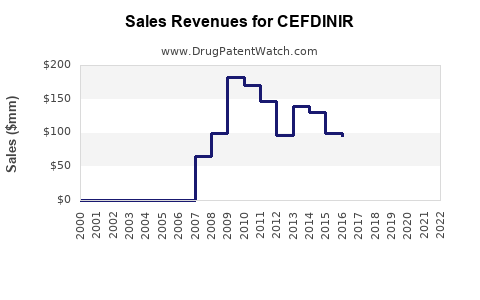
Over the past decade, Cefdinir has experienced stable demand, primarily in developed markets. Revenue figures align closely with antibiotic market trends, with growth driven by increased access to healthcare and pediatric vaccination programs reducing the incidence of complex infections. Generic availability has suppressed pricing, but volume-based growth has maintained revenue streams.
Projected Growth
The global Cefdinir market is expected to grow at a Compound Annual Growth Rate (CAGR) of approximately 3.2% from 2023 to 2028, with key growth contributors including:
- Emerging markets: Rapid urbanization and healthcare infrastructure development will expand access.
- Expanded indications: Off-label uses and longer treatment courses in certain infections could boost demand.
- Regulatory approvals: New formulations or combinations may extend patent life or open new segments.
Potential Risks to Financial Trajectory
- Antimicrobial stewardship policies: Stricter guidelines could limit Cefdinir prescribing, particularly for conditions where it is not first-line.
- Resistance patterns: Growing resistance could diminish efficacy, necessitating shifts to alternative therapies and impacting revenue.
- Competitive innovations: Introduction of newer antibiotics with improved efficacy or safety profiles may erode Cefdinir’s market share.
Strategic Implications
Pharmaceutical companies operating in this space should prioritize:
- Investing in surveillance and stewardship programs to preserve Cefdinir’s efficacy.
- Developing formulations that meet patient and clinician preferences, including pediatric-friendly options.
- Engaging with regulators and health authorities to align on guidelines that sustain appropriate Cefdinir use.
- Expanding geographic reach via partnerships and local manufacturing to capitalize on emerging market growth.
Conclusion
Cefdinir’s market remains resilient, supported by its clinical profile and ongoing demand in outpatient settings. Nevertheless, its future financial trajectory faces challenges from evolving prescribing practices, resistance, and competition from emerging antibiotics. Strategic adaptation—focused on stewardship, innovation, and geographic expansion—will determine long-term profitability.
Key Takeaways
- The global Cefdinir market is projected to grow modestly at 3.2% CAGR through 2028, driven by emerging markets and expanding indications.
- Competitive pressures, resistance development, and antimicrobial stewardship are the primary risks influencing Cefdinir’s market size and profitability.
- Patent expirations and generic proliferation exert downward pricing pressure but are offset by maintained volume demand.
- Regulatory focus on appropriate antibiotic use supports sustained demand in compliant markets, though overuse remains a concern.
- Strategic investments in formulation innovation, surveillance, and geographic expansion are critical for maintaining market relevance.
FAQs
-
What factors influence Cefdinir’s market growth?
Demand is primarily driven by the prevalence of bacterial respiratory infections, pediatric healthcare access, and prescribing habits. Emerging markets and expanding indications also contribute to growth.
-
How does antibiotic resistance impact Cefdinir?
Increasing resistance among key pathogens can reduce Cefdinir’s efficacy, leading to decreased prescribing and potential development of new formulations or combination therapies.
-
What regulations affect Cefdinir’s market?
Authorities emphasize antimicrobial stewardship, safety monitoring, and appropriate prescribing practices, all of which can influence market access and demand.
-
Are there upcoming competitors to Cefdinir?
Yes. Newer oral antibiotics like lefamulin and omadacycline could compete for certain indications, potentially affecting Cefdinir’s market share.
-
What strategies can pharmaceutical companies adopt to sustain Cefdinir’s market?
Emphasize stewardship programs, develop patient-friendly formulations, expand to emerging markets, and monitor resistance trends to adapt usage guidelines proactively.
References
[1] MarketsandMarkets. (2022). Antibiotics Market by Type, Application, and Region — Global Forecast to 2027.