ARISTADA INITIO KIT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Aristada Initio Kit, and what generic alternatives are available?
Aristada Initio Kit is a drug marketed by Alkermes Inc and is included in one NDA. There are nine patents protecting this drug.
This drug has one hundred and four patent family members in twenty-eight countries.
The generic ingredient in ARISTADA INITIO KIT is aripiprazole lauroxil. There are forty-nine drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the aripiprazole lauroxil profile page.
DrugPatentWatch® Generic Entry Outlook for Aristada Initio Kit
Aristada Initio Kit was eligible for patent challenges on October 5, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 8, 2035. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ARISTADA INITIO KIT?
- What are the global sales for ARISTADA INITIO KIT?
- What is Average Wholesale Price for ARISTADA INITIO KIT?
Summary for ARISTADA INITIO KIT
| International Patents: | 104 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 58 |
| Clinical Trials: | 2 |
| Patent Applications: | 265 |
| DailyMed Link: | ARISTADA INITIO KIT at DailyMed |
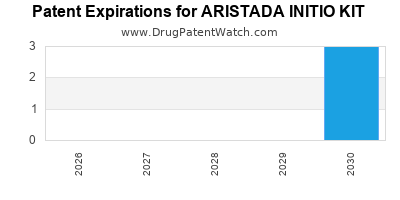
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ARISTADA INITIO KIT
Generic Entry Date for ARISTADA INITIO KIT*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ARISTADA INITIO KIT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of California, Los Angeles | Phase 4 |
| Alkermes, Inc. | Phase 4 |
| Alkermes, Inc. | Phase 3 |
Pharmacology for ARISTADA INITIO KIT
| Drug Class | Atypical Antipsychotic |
US Patents and Regulatory Information for ARISTADA INITIO KIT
ARISTADA INITIO KIT is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ARISTADA INITIO KIT is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkermes Inc | ARISTADA INITIO KIT | aripiprazole lauroxil | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 209830-001 | Jun 29, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Alkermes Inc | ARISTADA INITIO KIT | aripiprazole lauroxil | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 209830-001 | Jun 29, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Alkermes Inc | ARISTADA INITIO KIT | aripiprazole lauroxil | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 209830-001 | Jun 29, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for ARISTADA INITIO KIT
When does loss-of-exclusivity occur for ARISTADA INITIO KIT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 15306198
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017002926
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 57762
Estimated Expiration: ⤷ Get Started Free
China
Patent: 6794251
Estimated Expiration: ⤷ Get Started Free
Patent: 2494492
Estimated Expiration: ⤷ Get Started Free
Patent: 2641785
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0190642
Estimated Expiration: ⤷ Get Started Free
Patent: 0211271
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 21594
Estimated Expiration: ⤷ Get Started Free
Patent: 24625
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 43169
Estimated Expiration: ⤷ Get Started Free
Patent: 55711
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 0607
Estimated Expiration: ⤷ Get Started Free
Patent: 2079
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 71166
Estimated Expiration: ⤷ Get Started Free
Patent: 26167
Estimated Expiration: ⤷ Get Started Free
Patent: 57830
Estimated Expiration: ⤷ Get Started Free
Patent: 17524021
Estimated Expiration: ⤷ Get Started Free
Patent: 20007316
Estimated Expiration: ⤷ Get Started Free
Patent: 21059604
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1417
Patent: COMPOSICIONES DE PROFARMACOS DE ARIPIPRAZOL. (ARIPIPRAZOLE PRODRUG COMPOSITIONS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 17002029
Patent: COMPOSICIONES DE PROFARMACOS DE ARIPIPRAZOL. (ARIPIPRAZOLE PRODRUG COMPOSITIONS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8914
Patent: Aripiprazole prodrug compositions
Estimated Expiration: ⤷ Get Started Free
Patent: 7204
Patent: Aripiprazole prodrug compositions
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 05376
Patent: КОМПОЗИЦИИ ПРОЛЕКАРСТВА АРИПИПРАЗОЛА (ARIPIPRAZOLE PRODRUG COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 17108204
Patent: КОМПОЗИЦИИ ПРОЛЕКАРСТВА АРИПИПРАЗОЛА
Estimated Expiration: ⤷ Get Started Free
Patent: 19134055
Patent: КОМПОЗИЦИИ ПРОЛЕКАРСТВА АРИПИПРАЗОЛА
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01900236
Estimated Expiration: ⤷ Get Started Free
Patent: 02100481
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 746
Patent: KOMPOZICIJE PROLEKA ARIPIPRAZOLA (ARIPIPRAZOLE PRODRUG COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 232
Patent: KOMPOZICIJE PROLEKA ARIPIPRAZOLA (ARIPIPRAZOLE PRODRUG COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 82958
Estimated Expiration: ⤷ Get Started Free
Patent: 08196
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 21270
Estimated Expiration: ⤷ Get Started Free
Patent: 84849
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1905694
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ARISTADA INITIO KIT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2721270 | ⤷ Get Started Free | |
| New Zealand | 767204 | Aripiprazole prodrug compositions | ⤷ Get Started Free |
| European Patent Office | 4124616 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ARISTADA INITIO KIT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1675573 | 300669 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ARIPIPRAZOLE; REGISTRATION NO/DATE: EU/1/13/882 20131115 |
| 1675573 | C300669 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ARIPIPRAZOLE; REGISTRATION NO/DATE: EU/1/13/882 20131115 |
| 1675573 | 2014C/029 | Belgium | ⤷ Get Started Free | PRODUCT NAME: ARIPIPRAZOLE; AUTHORISATION NUMBER AND DATE: EU/1/13/882 20131119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: ARISTADA INITIO KIT
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
