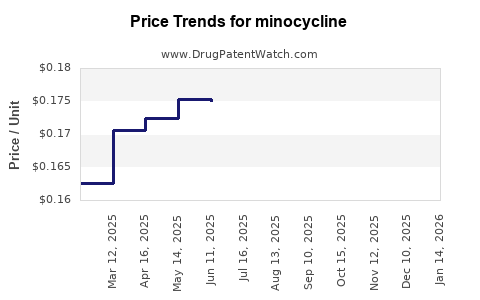
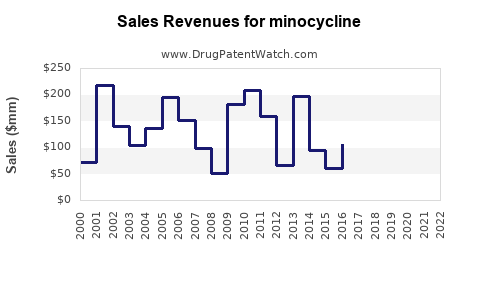
Minocycline - Generic Drug Details
✉ Email this page to a colleague
Generic filers with tentative approvals for MINOCYCLINE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Get Started Free | ⤷ Get Started Free | EQ 105MG BASE | Tablet, Extended Release; Oral |
| ⤷ Get Started Free | ⤷ Get Started Free | EQ 80MG BASE | Tablet, Extended Release; Oral |
| ⤷ Get Started Free | ⤷ Get Started Free | EQ 55MG BASE | TABLET, EXTENDED RELEASE; ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
US Patents and Regulatory Information for minocycline
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-005 | Jul 11, 2012 | DISCN | No | No | 7,790,705 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Sandoz | MINOCYCLINE HYDROCHLORIDE | minocycline hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 090422-003 | Aug 13, 2009 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Journey | XIMINO | minocycline hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 201922-003 | Jul 11, 2012 | DISCN | No | No | 8,252,776 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Sun Pharm | MINOCYCLINE HYDROCHLORIDE | minocycline hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 204394-006 | Oct 7, 2022 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Bausch | SOLODYN | minocycline hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 050808-007 | Aug 27, 2010 | DISCN | Yes | No | 8,252,776 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |