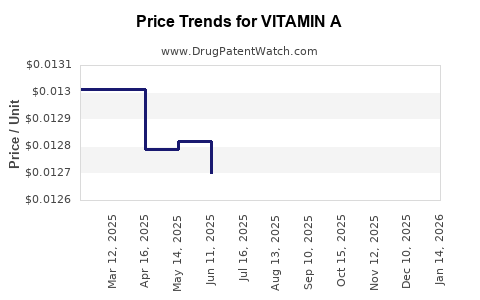
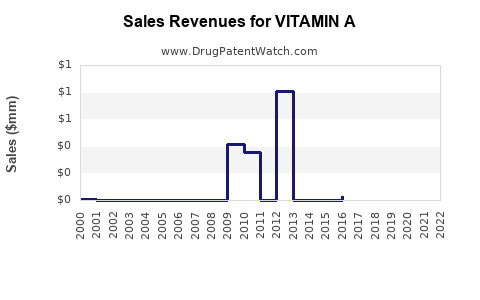
Last updated: July 27, 2025
Introduction
Vitamin A, a fat-soluble vitamin essential for vision, immune function, and cellular growth, has long been classified both as a dietary supplement and as a therapeutic agent in treating deficiency-related conditions. Its global market encompasses a broad spectrum of applications, including over-the-counter (OTC) supplements, fortification programs, and pharmaceutical formulations. As a vital nutrient with diverse therapeutic roles, understanding the current market dynamics and projecting its financial trajectory requires an analysis of supply and demand factors, regulatory landscapes, manufacturing complexities, and emerging therapeutic uses.
Historical Market Context and Current Standing
Vitamin A was first isolated in the early 20th century, with widespread recognition as a dietary supplement by mid-century. The global vitamin A market experienced rapid growth in response to increasing awareness of deficiency-related blindness and immune deficiencies, particularly in developing nations. According to recent reports, the global vitamin A market size was valued at approximately USD 1.2 billion in 2021 and is anticipated to grow at a compound annual growth rate (CAGR) of around 5% through 2028 [1].
While traditionally dominated by supplement and fortification segments, evolving science and novel therapeutic explorations are expanding the drug’s application spectrum. The market's evolution is influenced by socio-economic factors, governmental policies, and advances in bioavailability and synthesis technologies.
Market Drivers
Global Vitamin A Deficiency (VAD) Burden
VAD remains a significant public health challenge, particularly in low-income countries, with WHO estimates that about 19 million pre-school children are affected. This demand sustains both the supplement market and pharmaceutical interventions [2]. Fortification programs targeting staple foods such as sugar, dairy, and cooking oil further amplify demand.
Rising Awareness of Immune and Eye Health
Public awareness campaigns and scientific evidence supporting vitamin A’s role in immune health and ocular health—such as prevention of xerophthalmia and retinitis pigmentosa—fuel both OTC and prescription markets.
Expansion into Therapeutic Indications
Recent advances have seen vitamin A derivatives, notably retinoids, playing crucial roles in dermatology (e.g., acne management), oncology, and dermatological cancers. The development of pharmaceutical-grade vitamin A formulations and retinoid-based drugs opens new revenue streams and broadens the market scope.
Regulatory and Policy Support
Government-led programs for deficiency eradication and regulatory approval processes for therapeutic indications bolster market stability. For instance, UNICEF and WHO-backed supplementation initiatives stimulate sustained demand in emerging markets.
Market Challenges
Supply Chain and Manufacturing Complexities
Vitamin A’s production involves complex processes such as extraction from animal sources (liver), biosynthesis (beta-carotene conversion), or chemical synthesis (retinol production). Ensuring quality, purity, and bioavailability while managing costs imposes significant manufacturing hurdles. As a result, supply disruptions or regulatory scrutiny over contaminations can impact market stability.
Safety Concerns and Regulatory Constraints
Excessive intake of vitamin A can cause hypervitaminosis, leading to toxicity. Regulatory authorities such as FDA and EMA impose strict guidelines on overdosage, dosing in pharmaceuticals, and labeling, which can influence product development and market penetration.
Market Penetration in Developing Nations
Despite high demand, distribution challenges, lack of awareness, and affordability constraints in low-income countries limit impact, requiring cooperative efforts and investment to maximize market potential.
Emerging Trends and Innovations
Biofortification and Functional Foods
Developments in crop biofortification and genetically enhanced foods aim to alleviate VAD through dietary integration, potentially decreasing dependence on pharmaceutical interventions but creating competition within the market.
Nanoformulations and Enhancing Bioavailability
Innovations such as nanoemulsions and lipid-based delivery systems aim to improve bioavailability, leading to more efficient therapeutic delivery, lower dosages, and reduced side effects.
Retinoid Derivatives and Novel Therapeutics
Research into retinoid analogs with refined specificity and reduced toxicity opens avenues for new drug formulations targeting dermatological and oncological indications, potentially expanding market revenue streams.
Digital and Precision Medicine
Integration with digital health records and personalized medicine strategies may facilitate targeted supplementation and therapy, optimizing clinical outcomes and market segmentation.
Financial Trajectory and Market Forecast
The upward trajectory of the vitamin A market is underpinned by increasing demand from emerging economies, expanding therapeutic applications, and technological innovations. The supplement segment continues to dominate due to population growth and health awareness, while pharmaceuticals representing retinoids and derivatives are poised for accelerated growth owing to their expanding therapeutic indications.
Forecasts are optimistic: a CAGR of approximately 5% until 2028, with valuations projected to reach USD 1.7 billion by 2028 [1]. Regions such as Asia-Pacific, with high deficiency prevalence and growing healthcare infrastructure, are expected to be significant drivers of market expansion. In parallel, regulatory support and R&D investments are critical in navigating the evolving landscape.
Strategic Considerations for Stakeholders
- Manufacturers should invest in advanced synthesis technologies and quality assurance protocols to ensure product consistency and safety.
- Pharmaceutical developers must explore retinoid derivatives with improved safety profiles for dermatological and oncological indications.
- Policy advocates should promote fortification and supplementation programs aligned with scientific evidence.
- Investors should monitor R&D pipelines, regulatory developments, and emerging markets to identify high-growth opportunities.
Key Takeaways
- The global vitamin A market is projected to grow steadily, driven by deficiency mitigation programs and expanding therapeutic applications.
- Advances in bioavailability, nanoformulations, and retinoid derivatives present significant innovation opportunities.
- Supply chain resilience, safety regulations, and public awareness are pivotal factors influencing market stability.
- Emerging economies, especially within Asia-Pacific, are key growth markets with increasing demand.
- Strategic investments in technology, R&D, and policy engagement are essential for capturing future value.
Conclusion
Vitamin A remains integral to both nutritional health and pharmaceutical innovation. Its market's current momentum hinges on global health initiatives, scientific advancements, and a diversified application landscape. Stakeholders capable of navigating regulatory complexities, technological evolution, and market disparities will be poised to leverage significant financial opportunities in this vital sector.
FAQs
1. What are the primary therapeutic applications of pharmaceutical-grade vitamin A?
Vitamin A and its derivatives, such as retinoids, are used in ophthalmology to treat deficiency-related blindness, dermatology for acne and psoriasis, and oncology, particularly in differentiating erythroleukemia and certain skin cancers.
2. How does supply chain complexity impact the vitamin A market?
Supply chain challenges arise from sourcing raw materials (e.g., liver, carotenoids), manufacturing bottlenecks, and quality control issues, which can cause fluctuations in availability and price stability.
3. What are regulatory concerns associated with vitamin A?
Regulatory agencies monitor dosage limits to prevent toxicity, approve therapeutic indications, and oversee manufacturing practices. Excessive intake can lead to hypervitaminosis A, prompting strict regulation and labeling requirements.
4. How might emerging technologies influence vitamin A's market growth?
Innovations like nanoformulations enhance bioavailability, allowing lower doses and improved efficacy. Additionally, R&D into retinoid analogs can lead to safer, more targeted therapies, opening new revenue streams.
5. Which regions are expected to dominate the future vitamin A market?
Asia-Pacific is anticipated to lead due to high deficiency prevalence, expanding healthcare infrastructure, and increasing consumption of supplements and pharmaceuticals targeting VAD.
References
- MarketWatch. "Vitamin A Market Size, Share & Industry Analysis, 2021–2028."
- WHO. "Vitamin A Supplementation in Populations at Risk of Deficiency."