Formulation information for generic ingredient: abacavir sulfate
✉ Email this page to a colleague
ABACAVIR SULFATE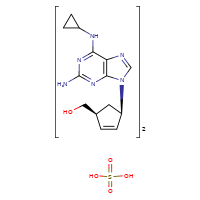
CASRN: 188062-50-2
Manufacturing Methods
…<truncated in preview>
You’re using a public version of DrugPatentWatch with 5 free searches available | Register to unlock more free searches. CREATE FREE ACCOUNT
Last Updated: April 26, 2024
✉ Email this page to a colleague

CASRN: 188062-50-2
<truncated in preview>